Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience
- PMID: 38543741
- PMCID: PMC10974793
- DOI: 10.3390/v16030375
Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience
Abstract
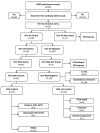
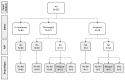
Injection drug use represents an important contributor to hepatitis C virus (HCV) transmission, hence therapeutic communities (TCs) are promising points of care for the identification and treatment of HCV-infected persons who inject drugs (PWIDs). We evaluated the effectiveness and efficacy of an HCV micro-elimination program targeting PWIDs in the context of a drug-free TC; we applied the cascade of care (CoC) evaluation by calculating frequencies of infection diagnosis, confirmation, treatment and achievement of a sustained virological response (SVR). We also evaluated the risk of reinfection of PWIDs achieving HCV eradication by collecting follow-up virologic information of previously recovered individuals and eventual relapse in drug use, assuming the latter as a potential source of reinfection. We considered 811 PWIDs (aged 18+ years) residing in San Patrignano TC at the beginning of the observation period (January 2018-March 2022) or admitted thereafter, assessing for HCV and HIV serology and viral load by standard laboratory procedures. Ongoing infections were treated with direct-acting antivirals (DAA), according to the current national guidelines. Out of the 792 individuals tested on admission, 503 (63.5%) were found to be seropositive for antibodies against HCV. A total of 481 of these 503 individuals (95.6%) underwent HCV RNA testing. Out of the 331 participants positive for HCV RNA, 225 were ultimately prescribed a DAA treatment with a sustained viral response (SVR), which was achieved by 222 PWIDs (98.7%). Of the 222 PWIDs, 186 (83.8%) with SVR remained HCV-free on follow-up (with a median follow-up of 2.73 years after SVR ascertainment). The CoC model in our TC proved efficient in implementing HCV micro-elimination, as well as in preventing reinfection and promoting retention in the care of individuals, which aligns with the therapeutic goals of addiction treatment.
Keywords: HCV; cascade of care; intravenous drug use; therapeutic community.
Conflict of interest statement
Antonio Boschini has received institutional grants from Gilead Science. None of the other authors have any conflicts of interest to report.
Figures

References
-
- Trickey A., Fraser H., Lim A.G., Peacock A., College S., Walker J.G., Leung J., Grebely J., Larney S., Martin N.K., et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019;4:435–444. doi: 10.1016/S2468-1253(19)30085-8. - DOI - PMC - PubMed
-
- Safreed-Harmon K., Blach S., Aleman S., Bollerup S., Cooke G., Dalgard O., Dillon J.F., Dore G.J., Duberg A.-S., Grebely J., et al. The Consensus Hepatitis C Cascade of Care: Standardized reporting to monitor progress toward elimination. Clin. Infect. Dis. 2019;69:2218–2227. doi: 10.1093/cid/ciz714. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

