Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review
- PMID: 38543764
- PMCID: PMC10975848
- DOI: 10.3390/v16030399
Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review
Abstract
Background: Dolutegravir (DTG) is a cornerstone of global antiretroviral (ARV) therapy (ART) due to its high efficacy and favorable tolerability. However, limited data exist regarding the risk of emergent integrase strand transfer inhibitor (INSTI) drug-resistance mutations (DRMs) in individuals receiving DTG-containing ART.
Methods: We performed a PubMed search using the term "Dolutegravir", last updated 18 December 2023, to estimate the prevalence of VF with emergent INSTI DRMs in people living with HIV (PLWH) without previous VF on an INSTI who received DTG-containing ART.
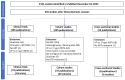
Results: Of 2131 retrieved records, 43 clinical trials, 39 cohorts, and 6 cross-sectional studies provided data across 6 clinical scenarios based on ART history, virological status, and co-administered ARVs: (1) ART-naïve PLWH receiving DTG plus two NRTIs; (2) ART-naïve PLWH receiving DTG plus lamivudine; (3) ART-experienced PLWH with VF on a previous regimen receiving DTG plus two NRTIs; (4) ART-experienced PLWH with virological suppression receiving DTG plus two NRTIs; (5) ART-experienced PLWH with virological suppression receiving DTG and a second ARV; and (6) ART-experienced PLWH with virological suppression receiving DTG monotherapy. The median proportion of PLWH in clinical trials with emergent INSTI DRMs was 1.5% for scenario 3 and 3.4% for scenario 6. In the remaining four trial scenarios, VF prevalence with emergent INSTI DRMs was ≤0.1%. Data from cohort studies minimally influenced prevalence estimates from clinical trials, whereas cross-sectional studies yielded prevalence data lacking denominator details.
Conclusions: In clinical trials, the prevalence of VF with emergent INSTI DRMs in PLWH receiving DTG-containing regimens has been low. Novel approaches are required to assess VF prevalence with emergent INSTI DRMs in PLWH receiving DTG in real-world settings.
Keywords: HIV; epidemiology; systematic review; treatment.
Conflict of interest statement
Robert W. Shafer has received honoraria for participation in advisory boards from Gilead Sciences and GlaxoSmithKline and speaking honoraria from Gilead Sciences and ViiV Healthcare.
Figures
References
-
- World Health Organization Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. [(accessed on 17 August 2022)]. Available online: https://www.who.int/publications-detail-redirect/9789240031593. - PubMed
-
- Clinton Health Access Initiative . The State of HIV Treatment, Testing, and Prevention in Low- and Middle-Income Countries. Clinton Health Access Initiative; Boston, MA, USA: 2023. HIV Market Report 2023.
-
- Tzou P.L., Rhee S.-Y., Descamps D., Clutter D.S., Hare B., Mor O., Grude M., Parkin N., Jordan M.R., Bertagnolio S., et al. Integrase Strand Transfer Inhibitor (INSTI)-Resistance Mutations for the Surveillance of Transmitted HIV-1 Drug Resistance. J. Antimicrob. Chemother. 2020;75:170–182. doi: 10.1093/jac/dkz417. - DOI - PMC - PubMed

