Natural Adeno-Associated Virus Serotypes and Engineered Adeno-Associated Virus Capsid Variants: Tropism Differences and Mechanistic Insights
- PMID: 38543807
- PMCID: PMC10975205
- DOI: 10.3390/v16030442
Natural Adeno-Associated Virus Serotypes and Engineered Adeno-Associated Virus Capsid Variants: Tropism Differences and Mechanistic Insights
Erratum in
-
Correction: Lopez-Gordo et al. Natural Adeno-Associated Virus Serotypes and Engineered Adeno-Associated Virus Capsid Variants: Tropism Differences and Mechanistic Insights. Viruses 2024, 16, 442.Viruses. 2024 Aug 28;16(9):1366. doi: 10.3390/v16091366. Viruses. 2024. PMID: 39339983 Free PMC article.
Abstract
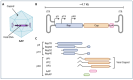
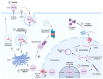
Today, adeno-associated virus (AAV)-based vectors are arguably the most promising in vivo gene delivery vehicles for durable therapeutic gene expression. Advances in molecular engineering, high-throughput screening platforms, and computational techniques have resulted in a toolbox of capsid variants with enhanced performance over parental serotypes. Despite their considerable promise and emerging clinical success, there are still obstacles hindering their broader use, including limited transduction capabilities, tissue/cell type-specific tropism and penetration into tissues through anatomical barriers, off-target tissue biodistribution, intracellular degradation, immune recognition, and a lack of translatability from preclinical models to clinical settings. Here, we first describe the transduction mechanisms of natural AAV serotypes and explore the current understanding of the systemic and cellular hurdles to efficient transduction. We then outline progress in developing designer AAV capsid variants, highlighting the seminal discoveries of variants which can transduce the central nervous system upon systemic administration, and, to a lesser extent, discuss the targeting of the peripheral nervous system, eye, ear, lung, liver, heart, and skeletal muscle, emphasizing their tissue and cell specificity and translational promise. In particular, we dive deeper into the molecular mechanisms behind their enhanced properties, with a focus on their engagement with host cell receptors previously inaccessible to natural AAV serotypes. Finally, we summarize the main findings of our review and discuss future directions.
Keywords: AAV engineering; AAV receptor; CNS; brain-blood barrier; directed evolution.
Conflict of interest statement
Thomas Weber is an employee of Spark Therapeutics. The other authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

