Safety Monitoring of COVID-19 Vaccines in Persons with Prior SARS-CoV-2 Infection: A European Multi-Country Study
- PMID: 38543875
- PMCID: PMC10974422
- DOI: 10.3390/vaccines12030241
Safety Monitoring of COVID-19 Vaccines in Persons with Prior SARS-CoV-2 Infection: A European Multi-Country Study
Abstract
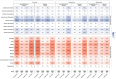
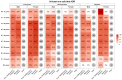
In all pivotal trials of COVID-19 vaccines, the history of previous SARS-CoV-2 infection was mentioned as one of the main exclusion criteria. In the absence of clinical trials, observational studies are the primary source for evidence generation. This study aims to describe the patient-reported adverse drug reactions (ADRs) following the first COVID-19 vaccination cycle, as well as the administration of booster doses of different vaccine brands, in people with prior SARS-CoV-2 infection, as compared to prior infection-free matched cohorts of vaccinees. A web-based prospective study was conducted collecting vaccinee-reported outcomes through electronic questionnaires from eleven European countries in the period February 2021-February 2023. A baseline questionnaire and up to six follow-up questionnaires collected data on the vaccinee's characteristics, as well as solicited and unsolicited adverse reactions. Overall, 3886 and 902 vaccinees with prior SARS-CoV-2 infection and having received the first dose or a booster dose, respectively, were included in the analysis. After the first dose or booster dose, vaccinees with prior SARS-CoV-2 infection reported at least one ADR at a higher frequency than those matched without prior infection (3470 [89.6%] vs. 2916 [75.3%], and 614 [68.2%] vs. 546 [60.6%], respectively). On the contrary side, after the second dose, vaccinees with a history of SARS-CoV-2 infection reported at least one ADR at a lower frequency, compared to matched controls (1443 [85.0%] vs. 1543 [90.9%]). The median time to onset and the median time to recovery were similar across all doses and cohorts. The frequency of adverse reactions was higher in individuals with prior SARS-CoV-2 infection who received Vaxzevria as the first dose and Spikevax as the second and booster doses. The frequency of serious ADRs was low for all doses and cohorts. Data from this large-scale prospective study of COVID-19 vaccinees could be used to inform people as to the likelihood of adverse effects based on their history of SARS-CoV-2 infection, age, sex, and the type of vaccine administered. In line with pivotal trials, the safety profile of COVID-19 vaccines was also confirmed in people with prior SARS-CoV-2 infection.
Keywords: COVID-19 vaccines; Covid Vaccine Monitor; active surveillance; adverse event; people with prior SARS-CoV-2 infection.
Conflict of interest statement
Miriam Sturkenboom is head of a department that conducts studies for regulatory agencies and pharmaceutical companies, with research grants to the institution; this includes Pfzer, Janssen and AstraZeneca. All studies are conducted using the ENCePP code of conduct. Gianluca Trifrò has served in the last three years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead, Amgen; he was the scientifc director of a Master’s program on pharmacovigilance, pharmacoepidemiology and real-world evidence which has received a non-conditional grant from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also the scientifc coordinator of the academic spin-of “INSPIRE srl” which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). None of these listed activities is related to the topic of the manuscript. Francesco Ciccimarra, Nicoletta Luxi, Chiara Bellitto, Luca L’Abbate, Monika Raethke, Florence van Hunsel, Thomas Lieber, Erik Mulder, Fabio Riefolo, Caroline Dureau-Pournin, Andreea Farcas, Francisco Batel Marques, Kathryn Morton, Debabrata Roy, Simona Sonderlichová, Nicolas H. Thurin, Felipe Villalobos have no conficts of interest that are directly relevant to the content of this article.
Figures




References
-
- COVID-19 Vaccines: Key Facts. Human Regulatory. [(accessed on 7 February 2024)]. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr....
-
- Luxi N., Giovanazzi A., Capuano A., Crisafulli S., Cutroneo P.M., Fantini M.P., Ferrajolo C., Moretti U., Poluzzi E., Raschi E., et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021;44:1247–1269. doi: 10.1007/s40264-021-01131-6. - DOI - PMC - PubMed
-
- COVID-19 Vaccines: Development, Evaluation, Approval and Monitoring. Human Regulatory. [(accessed on 7 February 2024)]. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

