Prolonged SARS-CoV-2 T Cell Responses in a Vaccinated COVID-19-Naive Population
- PMID: 38543904
- PMCID: PMC10976022
- DOI: 10.3390/vaccines12030270
Prolonged SARS-CoV-2 T Cell Responses in a Vaccinated COVID-19-Naive Population
Abstract
Introduction: Exploring T cell response duration is pivotal for understanding immune protection evolution in natural SARS-CoV-2 infections. The objective of the present study was to analyze the T cell immune response over time in individuals who were both vaccinated and COVID-19-naive and had undetectable levels of SARS-CoV-2 IgG antibodies at the time of testing.
Methods: We performed a retrospective descriptive analysis using data extracted from the electronic medical records of consecutive adult individuals who underwent COVID-19 immunity screening at a private healthcare center from September 2021 to September 2022. The study participants were divided into three groups according to the post-vaccination time period, as follows: group A (up to 3 months), group B (3-6 months), and group C (>6 months). T cell response was evaluated using the IGRA methodology T-SPOT®.COVID.
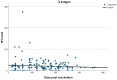
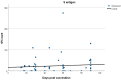
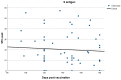
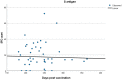
Results: Of the total number of subjects (n = 165), 60/165 (36.4%) had been vaccinated in the last 3 months (group A), 57/165 (34.5%) between 3 and 6 months (group B), and 48/165 (29.1%) at least 6 months prior to the examination day (group C). T cell positivity was reported in 33/60 (55.0%) of group A, 45/57 (78.9%) of group B, and 36/48 (75%) of group C (p < 0.007). No statistically significant differences were revealed in the spot-forming cell (SFC) count among groups, with mean SFC counts of 75.96 for group A, 89.92 for group B, and 83.58 for group C (Kruskal-Wallis test, p = 0.278).
Conclusions: Our findings suggest that cellular immunity following SARS-CoV-2 vaccination may endure for at least six months, even in the presence of declining or absent IgG antibody levels.
Keywords: COVID-19; SARS-CoV-2; T cell immunity; cellular immunity; vaccination; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gil-Manso S., Carbonell D., Lopez-Fernandez L., Miguens I., Alonso R., Buno I., Munoz P., Ochando J., Pion M., Correa-Rocha R. Induction of High Levels of Specific Humoral and Cellular Responses to SARS-CoV-2 After the Administration of COVID-19 mRNA Vaccines Requires Several Days. Front. Immunol. 2021;12:726960. doi: 10.3389/fimmu.2021.726960. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

