Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses
- PMID: 38543913
- PMCID: PMC10975332
- DOI: 10.3390/vaccines12030279
Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses
Abstract
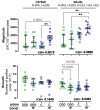
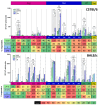
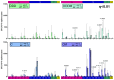
The efficacy of anti-viral T-cell vaccines may greatly depend on their ability to generate high-magnitude responses targeting a broad range of different epitopes. Recently, we created the HIV T-cell immunogen HTI, designed to generate T-cell responses to protein fragments more frequently targeted by HIV controllers. In the present study, we aim to maximize the breadth and magnitude of the T-cell responses generated by HTI by combining different vaccine vectors expressing HTI. We evaluated the ability to induce strong and broad T-cell responses to the HTI immunogen through prime vaccination with DNA plasmid (D) or Chimpanzee Adenovirus Ox1 (ChAdOx1; C) vectors, followed by a Modified Virus Ankara (MVA; M) vaccine boost (DDD, DDDM, C, and CM). HTI-specific T-cell responses after vaccination were measured by IFN-γ-ELISpot assays in two inbred mice strains (C57BL/6 and BALB/c). CM was the schedule triggering the highest magnitude of the response in both mice strains. However, this effect was not reflected in an increase in the breadth of the response but rather in an increase in the magnitude of the response to specific immunodominant epitopes. Immunodominance profiles in the two mouse strains were different, with a clear dominance of T-cell responses to a Pol-derived peptide pool after CM vaccination in C57BL/6. Responses to CM vaccination were also maintained at higher magnitudes over time (13 weeks) compared to other vaccination regimens. Thus, while a ChAdOx1 prime combined with MVA booster vaccination generated stronger and more sustained T-cell responses compared to three DNA vaccinations, the ChAdOx1 primed responses were more narrowly targeted. In conclusion, our findings suggest that the choice of vaccine vectors and prime-boost regimens plays a crucial role in determining the strength, duration, breadth, and focus of T-cell responses, providing further guidance for selecting vaccination strategies.
Keywords: HIV; T-cell vaccine; immune memory; immunodominance.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. BM and CB are co-inventors of the HTI immunogen (patent application PCT/EP2013/051596), co-inventor of vaccine regimens (US patent Application No. 62/935,519 and US Appl. No. 62/851,546) which may have relevance to this study. BM reports consultancy fees from AELIX Therapeutics; advisory and speaker fees from Gilead, Janssen, ViiV outside the submitted work. CB is co-founder, CSO and shareholder of AELIX Therapeutics. All other authors declare no conflicts of interest.
Figures




Similar articles
-
Priming with Recombinant BCG Expressing HTI Enhances the Magnitude and Breadth of the T-Cell Immune Responses Elicited by MVA.HTI in BALB/c Mice.Vaccines (Basel). 2020 Nov 13;8(4):678. doi: 10.3390/vaccines8040678. Vaccines (Basel). 2020. PMID: 33202884 Free PMC article.
-
Recombinant BCG Expressing HTI Prime and Recombinant ChAdOx1 Boost Is Safe and Elicits HIV-1-Specific T-Cell Responses in BALB/c Mice.Vaccines (Basel). 2019 Aug 2;7(3):78. doi: 10.3390/vaccines7030078. Vaccines (Basel). 2019. PMID: 31382453 Free PMC article.
-
Vaccination with an HIV T-Cell Immunogen (HTI) Using DNA Primes Followed by a ChAdOx1-MVA Boost Is Immunogenic in Gut Microbiota-Depleted Mice despite Low IL-22 Serum Levels.Vaccines (Basel). 2023 Oct 30;11(11):1663. doi: 10.3390/vaccines11111663. Vaccines (Basel). 2023. PMID: 38005995 Free PMC article.
-
Heterologous prime-boost regimens using rAd35 and rMVA vectors elicit stronger cellular immune responses to HIV proteins than homologous regimens.PLoS One. 2012;7(9):e45840. doi: 10.1371/journal.pone.0045840. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049876 Free PMC article.
-
Development of a DNA-MVA/HIVA vaccine for Kenya.Vaccine. 2002 May 6;20(15):1995-8. doi: 10.1016/s0264-410x(02)00085-3. Vaccine. 2002. PMID: 11983261 Review.
Cited by
-
Developing the next-generation of adenoviral vector vaccines.Hum Vaccin Immunother. 2025 Dec;21(1):2514356. doi: 10.1080/21645515.2025.2514356. Epub 2025 Jul 1. Hum Vaccin Immunother. 2025. PMID: 40590260 Free PMC article. Review.
-
Prospects for therapeutic T-cell vaccine strategies for HIV cure.Curr Opin HIV AIDS. 2025 Sep 1;20(5):463-471. doi: 10.1097/COH.0000000000000965. Epub 2025 Jul 9. Curr Opin HIV AIDS. 2025. PMID: 40638102 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

