Evaluation of Two Vaccines against Foot-and-Mouth Disease Used in Transcaucasian Countries by Small-Scale Immunogenicity Studies Conducted in Georgia, Azerbaijan and Armenia
- PMID: 38543929
- PMCID: PMC10975580
- DOI: 10.3390/vaccines12030295
Evaluation of Two Vaccines against Foot-and-Mouth Disease Used in Transcaucasian Countries by Small-Scale Immunogenicity Studies Conducted in Georgia, Azerbaijan and Armenia
Abstract
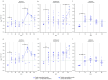
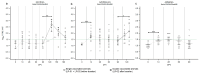
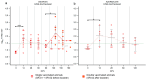
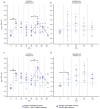
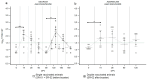
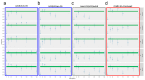
In countries endemic for foot-and-mouth disease (FMD), routine or emergency vaccinations are strategic tools to control the infection. According to the WOAH/FAO guidelines, a prior estimation of vaccine effectiveness is recommendable to optimize control programs. This study reports the results of a small-scale immunogenicity study performed in Transcaucasian Countries. Polyvalent vaccines, including FMDV serotypes O, A (two topotypes) and Asia1 from two different manufacturers, were evaluated in Georgia, Azerbaijan and Armenia. Naïve large and small ruminants were vaccinated once and a subgroup received a second booster dose. The titers of neutralizing antibodies in sera collected sequentially up to 180 DPV were determined through the Virus Neutralization Test versus homologous strains. This study led to the estimate that both the vaccines evaluated will not induce a protective and long-lasting population immunity, even after a second vaccination, stressing that consecutive administrations of both vaccines every three months are mandatory if one aspires to achieve protective herd immunity.
Keywords: foot-and-mouth disease; small-scale immunogenicity study; vaccination campaign; vaccine effectiveness assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation of the effectiveness of foot-and-mouth disease vaccination of animals in the buffer zone of the Republic of Armenia in 2016-2020.BMC Vet Res. 2023 Sep 29;19(1):176. doi: 10.1186/s12917-023-03728-8. BMC Vet Res. 2023. PMID: 37773157 Free PMC article.
-
Targeted FMD Vaccines for Eastern Africa: The AgResults Foot and Mouth Disease Vaccine Challenge Project.Viruses. 2021 Sep 14;13(9):1830. doi: 10.3390/v13091830. Viruses. 2021. PMID: 34578411 Free PMC article. Review.
-
The effects of simultaneous foot-and-mouth disease and Escherichia coli vaccination on the immunity of pregnant cows and their calves.Prev Vet Med. 2022 Jul;204:105645. doi: 10.1016/j.prevetmed.2022.105645. Epub 2022 Apr 6. Prev Vet Med. 2022. PMID: 35453090
-
Versatility of the adenovirus-vectored foot-and-mouth disease vaccine platform across multiple foot-and-mouth disease virus serotypes and topotypes using a vaccine dose representative of the AdtA24 conditionally licensed vaccine.Vaccine. 2018 Nov 19;36(48):7345-7352. doi: 10.1016/j.vaccine.2018.10.031. Epub 2018 Oct 13. Vaccine. 2018. PMID: 30327212
-
Global Foot-and-Mouth Disease Research Update and Gap Analysis: 3 - Vaccines.Transbound Emerg Dis. 2016 Jun;63 Suppl 1:30-41. doi: 10.1111/tbed.12521. Transbound Emerg Dis. 2016. PMID: 27320164 Review.
References
-
- FAO . Foot-and-Mouth Disease European Commission for the Control of Foot-and-Mouth Disease October–December 2021 Quarterly Report. FAO; Rome, Italy: 2022. 47p
-
- FAO . Foot-and-Mouth Disease European Commission for the Control of Foot-and-Mouth Disease January–March 2020 Quarterly Report. FAO; Rome, Italy: 2020. 48p
LinkOut - more resources
Full Text Sources

