Long-Term Immunological Alertness and Response to COVID-19 Vaccination-Conditions for Prevention in Early Palliative Oncological Care Patients
- PMID: 38543933
- PMCID: PMC10975612
- DOI: 10.3390/vaccines12030299
Long-Term Immunological Alertness and Response to COVID-19 Vaccination-Conditions for Prevention in Early Palliative Oncological Care Patients
Abstract
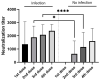
Aside from the general population, the COVID-19 pandemic has also affected a group of patients in palliative oncology care. In this study, long-term immune responses against SARS-CoV-2 after vaccination were monitored in a cohort of patients in palliative oncology care. This non-randomized, prospective, and open-label pilot study recruited patients from the Palliative Oncology Program and included 147 patients, of which 80 were females (54.4%) and 67 males (45.6%). The overall evaluation included current health status, SARS-CoV-2 anti-S IgG titer, and neutralizing antibodies using the SARS-CoV-2 virus neutralization test (VNT). Anti-S IgG antibody analysis revealed high (H) antibody levels in 35.7% (n = 10) and very high (VH) levels in 39.3% (n = 11) of patients after the second vaccination dose. Similarly, after the third dose, H was found in 29.6% (n = 32) and VH in 55.5% (n = 60) of patients. High and very high anti-S IgG antibody levels were consistent with high VNT titers (>2560) and H antibody levels in 17.1% (n = 12) or VH in 82.9% (n = 58) of patients. Patients with two or more doses showed H and VH antibody levels at a median of 451 and 342 days after vaccination, respectively. In this clinical trial, patients showed high and very high levels of anti-S IgG antibodies over a longer period of time. These patients did not show reduced immunological responses to the COVID-19 vaccine challenge. We can assume that prevention through vaccination can reduce the risk of complications or death from COVID-19 in patients in early palliative oncology care.
Keywords: COVID-19 vaccine; VNT; anti-S IgG; inpatient care; palliative oncological care.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Shedding of SARS-CoV-2 Virus in COVID-19 Patients and Neutralizing Antibody Level].Mikrobiyol Bul. 2022 Jul;56(3):416-431. doi: 10.5578/mb.20229704. Mikrobiyol Bul. 2022. PMID: 35960235 Turkish.
-
Assessment of antibody dynamics and neutralizing activity using serological assay after SARS-CoV-2 infection and vaccination.PLoS One. 2023 Sep 19;18(9):e0291670. doi: 10.1371/journal.pone.0291670. eCollection 2023. PLoS One. 2023. PMID: 37725623 Free PMC article.
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Neutralizing antibody titers six months after Comirnaty vaccination: kinetics and comparison with SARS-CoV-2 immunoassays.Clin Chem Lab Med. 2021 Dec 16;60(3):456-463. doi: 10.1515/cclm-2021-1247. Print 2022 Feb 23. Clin Chem Lab Med. 2021. PMID: 34911170
References
-
- Mohseni Afshar Z., Hosseinzadeh R., Barary M., Ebrahimpour S., Alijanpour A., Sayad B., Hosseinzadeh D., Rouhollah Miri S., Sio T.T., Sullman J.M.S., et al. Chalanges posed by COVID-19 in cancer patients: A narrative review. Cancer Med. 2022;11:1119–1135. doi: 10.1002/cam4.4519. - DOI - PMC - PubMed
-
- Bakitas M.A., Tosteson T.D., Li Z., Lyons K.D., Hull J.G., Li Z., Dione-Odom N., Frost J., Dragnev H.K., Hegel T.M., et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III Randomized controlled trial. Clin. Oncol. 2015;33:1438–1445. doi: 10.1200/JCO.2014.58.6362. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous