Influenza a Neuraminidase-Based Bivalent mRNA Vaccine Induces Th1-Type Immune Response and Provides Protective Effects in Mice
- PMID: 38543934
- PMCID: PMC10974139
- DOI: 10.3390/vaccines12030300
Influenza a Neuraminidase-Based Bivalent mRNA Vaccine Induces Th1-Type Immune Response and Provides Protective Effects in Mice
Abstract
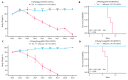
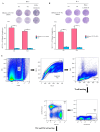
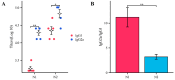
Vaccines are one of the most effective means of preventing influenza A, typically containing the hemagglutinin (HA) of the influenza A virus. However, antigenic drift and shift of the influenza A virus can lead to instability in vaccine efficacy. Compared to HA, the antigenic variation rate of neuraminidase (NA) is slower. In traditional inactivated influenza vaccines, although they contain a certain amount of NA, there are significant differences between different batches, which cannot consistently induce NA-based immune responses. Therefore, NA is often overlooked in vaccine development. In this study, we report an mRNA vaccine encoding the NA of two strains of influenza A virus. The experimental results demonstrated that when matched with the viral strain, this mRNA vaccine induced high levels of neutralizing antibodies, providing a protective effect to mice in viral challenge experiments, and this immune response was shown to be biased towards the Th1 type. In summary, this study demonstrates that NA is a promising potential antigen, providing new insights for the development of influenza A virus vaccines.
Keywords: Th1; influenza A; mRNA vaccine; neuraminidase.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



Similar articles
-
A Study on the Induction of Multi-Type Immune Responses in Mice via an mRNA Vaccine Based on Hemagglutinin and Neuraminidase Antigen.Vaccines (Basel). 2025 Jan 19;13(1):91. doi: 10.3390/vaccines13010091. Vaccines (Basel). 2025. PMID: 39852870 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Contribution of Neuraminidase to the Efficacy of Seasonal Split Influenza Vaccines in the Ferret Model.J Virol. 2022 Mar 23;96(6):e0195921. doi: 10.1128/jvi.01959-21. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107371 Free PMC article.
-
Influenza B virus neuraminidase: a potential target for next-generation vaccines?Expert Rev Vaccines. 2024 Jan-Dec;23(1):39-48. doi: 10.1080/14760584.2023.2290691. Epub 2023 Dec 14. Expert Rev Vaccines. 2024. PMID: 38037386 Review.
-
Towards a universal influenza vaccine: different approaches for one goal.Virol J. 2018 Jan 19;15(1):17. doi: 10.1186/s12985-017-0918-y. Virol J. 2018. PMID: 29370862 Free PMC article. Review.
Cited by
-
Improved influenza vaccine responses after expression of multiple viral glycoproteins from a single mRNA.Nat Commun. 2024 Oct 8;15(1):8712. doi: 10.1038/s41467-024-52940-z. Nat Commun. 2024. PMID: 39379405 Free PMC article.
-
A capless hairpin-protected mRNA vaccine encoding the full-length Influenza A hemagglutinin protects mice against a lethal Influenza A infection.Gene Ther. 2025 Jul;32(4):349-358. doi: 10.1038/s41434-025-00521-0. Epub 2025 Feb 23. Gene Ther. 2025. PMID: 39988620 Free PMC article.
-
A promising mRNA vaccine derived from the JN.1 spike protein confers protective immunity against multiple emerged Omicron variants.Mol Biomed. 2025 Mar 4;6(1):13. doi: 10.1186/s43556-025-00258-7. Mol Biomed. 2025. PMID: 40035925 Free PMC article.
-
A Study on the Induction of Multi-Type Immune Responses in Mice via an mRNA Vaccine Based on Hemagglutinin and Neuraminidase Antigen.Vaccines (Basel). 2025 Jan 19;13(1):91. doi: 10.3390/vaccines13010091. Vaccines (Basel). 2025. PMID: 39852870 Free PMC article.
References
-
- Hartvickson R., Cruz M., Ervin J., Brandon D., Forleo-Neto E., Dagnew A.F., Chandra R., Lindert K., Mateen A.A. Non-inferiority of mammalian cell-derived quadrivalent subunit influenza virus vaccines compared to trivalent subunit influenza virus vaccines in healthy children: A phase III randomized, multicenter, double-blind clinical trial. Int. J. Infect. Dis. 2015;41:65–72. doi: 10.1016/j.ijid.2015.11.004. - DOI - PubMed
Grants and funding
- 2021-I2M-1-043/CAMS Innovation Fund for Medical Sciences
- 202302AA310002/Major Science and Technology Special Program of Yunnan Provincial Department of Science and Technology
- 2022IMBCAMS004/Key Scientific Research Program of Institute of Medical Biology, Chinese Academy of Medical Sciences
- 202301AU070048/Special Program for Basic Research of Yunnan Provincial Department of Science and Technology
LinkOut - more resources
Full Text Sources

