Evaluation of Long-Term Adaptive Immune Responses Specific to SARS-CoV-2: Effect of Various Vaccination and Omicron Exposure
- PMID: 38543935
- PMCID: PMC10974805
- DOI: 10.3390/vaccines12030301
Evaluation of Long-Term Adaptive Immune Responses Specific to SARS-CoV-2: Effect of Various Vaccination and Omicron Exposure
Abstract
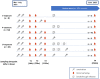
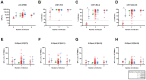
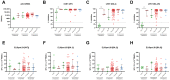
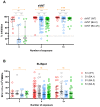
The immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) becomes increasingly complex as individuals receive different combinations of vaccine doses and encounter breakthrough infections. Our study focused on the immunogenicity observed over a two-year period in healthy individuals who completed a two-dose series and then experienced booster and/or Omicron infection. In June 2023, we recruited 78 healthcare workers who had previously participated in clinical research initiated in March 2021 at a single medical center in South Korea. At 1, 5, 11, and 25 months after a second dose, we assessed SARS-CoV-2-specific humoral and cellular immune responses. Longitudinal monitoring revealed a significant decline in humoral immunity levels after the second vaccine dose, followed by a substantial increase post-third vaccination or breakthrough infection. In contrast, stable cellular immune responses were consistently observed, with peak humoral and cellular immune measures reached at 25 months after the second dose. Among infection-naïve participants, three-dose vaccinated individuals had decreased neutralizing activity against wild-type (WT) and negative activities against Omicron subvariants BA.2 and BA.4/5, whereas those who received a fourth dose of bivalent BNT had significantly increased neutralizing activity (p < 0.05). All immune metrics tended to increase as the number of vaccine doses increased. Among participants with 4-exposure, homologous vaccination (mRNA × 4) led to higher humoral immunity, whereas heterologous vaccination (ChAd × 2/mRNA × 2) induced stronger cellular responses against multiple SARS-CoV-2 variants by enzyme-linked immunospot assays (p < 0.05). Immune responses from bivalent vaccines or Omicron infection did not show statistically significant differences among exposure number-matched participants (p > 0.05). Omicron exposure significantly increased cross-neutralizing activity, but magnitude of cellular immunity was not significantly altered by Omicron exposure. Our longitudinal study highlights the evolving complexity of SARS-CoV-2 immune responses, showing enhanced immunity with multiple vaccine doses and robust cellular responses from heterologous vaccination. These findings emphasize the need for ongoing surveillance to optimize vaccination strategies against emerging variants.
Keywords: Omicron; SARS-CoV-2; breakthrough infection; cellular immunity; humoral immunity; long-term; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ssentongo P., Ssentongo A.E., Voleti N., Groff D., Sun A., Ba D.M., Nunez J., Parent L.J., Chinchilli V.M., Paules C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022;22:439. doi: 10.1186/s12879-022-07418-y. - DOI - PMC - PubMed
-
- Pusnik J., Monzon-Posadas W.O., Zorn J., Peters K., Baum M., Proksch H., Schluter C.B., Alter G., Menting T., Streeck H. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 2023;14:572. doi: 10.1038/s41467-023-36250-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

