Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge
- PMID: 38543944
- PMCID: PMC10974367
- DOI: 10.3390/vaccines12030310
Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge
Abstract
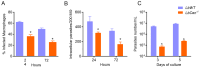
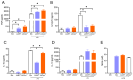
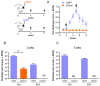
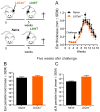
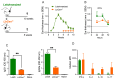
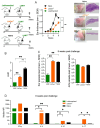
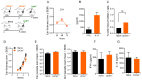
Immunization with various Leishmania species lacking centrin induces robust immunity against a homologous and heterologous virulent challenge, making centrin mutants a putative candidate for a leishmaniasis vaccine. Centrin is a calcium-binding cytoskeletal protein involved in centrosome duplication in higher eukaryotes and Leishmania spp. lacking centrin are unable to replicate in vivo and are non-pathogenic. We developed a centrin-deficient Leishmania braziliensis (LbCen-/-) cell line and confirmed its impaired survival following phagocytosis by macrophages. Upon experimental inoculation into BALB/c mice, LbCen-/- failed to induce lesions and parasites were rapidly eliminated. The immune response following inoculation with LbCen-/- was characterized by a mixed IFN-γ, IL-4, and IL-10 response and did not confer protection against L. braziliensis infection, distinct from L. major, L. donovani, and L mexicana centrin-deficient mutants. A prime-boost strategy also did not lead to a protective immune response against homologous challenge. On the contrary, immunization with centrin-deficient L. donovani (LdonCen-/-) cross-protected against L. braziliensis challenge, illustrating the ability of LdonCen-/- to induce the Th1-dominant protective immunity needed for leishmaniasis control. In conclusion, while centrin deficiency in L. braziliensis causes attenuation of virulence, and disrupts the ability to cause disease, it fails to stimulate a protective immune response.
Keywords: cutaneous leishmaniasis; immunization; live attenuated vaccine; vaccination.
Conflict of interest statement
Our contributions are an informal communication and represent our own best judgement. These comments do not bind or obligate the FDA.
Figures

Similar articles
-
Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing.Front Cell Infect Microbiol. 2022 Feb 17;11:790418. doi: 10.3389/fcimb.2021.790418. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35252020 Free PMC article.
-
Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis.J Immunol. 2009 Aug 1;183(3):1813-20. doi: 10.4049/jimmunol.0900276. Epub 2009 Jul 10. J Immunol. 2009. PMID: 19592661
-
Centrin-deficient Leishmania mexicana confers protection against Old World visceral leishmaniasis.NPJ Vaccines. 2022 Dec 3;7(1):157. doi: 10.1038/s41541-022-00574-x. NPJ Vaccines. 2022. PMID: 36463228 Free PMC article.
-
Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis.Vaccine. 2014 Jun 30;32(31):3895-901. doi: 10.1016/j.vaccine.2014.05.009. Epub 2014 May 14. Vaccine. 2014. PMID: 24837513 Review.
-
The History of Live Attenuated Centrin Gene-Deleted Leishmania Vaccine Candidates.Pathogens. 2022 Apr 2;11(4):431. doi: 10.3390/pathogens11040431. Pathogens. 2022. PMID: 35456106 Free PMC article. Review.
Cited by
-
Advances in Leishmania Vaccines: Current Development and Future Prospects.Pathogens. 2024 Sep 20;13(9):812. doi: 10.3390/pathogens13090812. Pathogens. 2024. PMID: 39339003 Free PMC article. Review.
References
-
- Pan American Health Organization LEISHMANIASES Epidemiological Report on the Region of the Americas. [(accessed on 2 February 2023)]. Available online: https://iris.paho.org/bitstream/handle/10665.2/56831/PAHOCDEVT220021_eng....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

