Spherical Silver Nanoparticles Located on Reduced Graphene Oxide Nanocomposites as Sensitive Electrochemical Sensors for Detection of L-Cysteine
- PMID: 38544052
- PMCID: PMC10973997
- DOI: 10.3390/s24061789
Spherical Silver Nanoparticles Located on Reduced Graphene Oxide Nanocomposites as Sensitive Electrochemical Sensors for Detection of L-Cysteine
Abstract
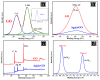
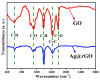
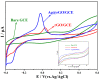
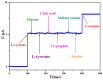
A new, simple, and effective one-step reduction method was applied to prepare a nanocomposite with spherical polycrystalline silver nanoparticles attached to the surface of reduced graphene oxide (Ag@rGO) at room temperature. Equipment such as X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared spectroscopy (FTIR) was used to characterize the morphology and composition of the Ag@rGO nanocomposite. A novel electrochemical sensor for detecting L-cysteine was proposed based on fixing Ag@rGO onto a glassy carbon electrode. The electrocatalytic behavior of the sensor was studied via cyclic voltammetry and amperometry. The results indicate that due to the synergistic effect of graphene with a large surface area, abundant active sites, and silver nanoparticles with good conductivity and high catalytic activity, Ag@rGO nanocomposites exhibit significant electrocatalytic activity toward L-cysteine. Under optimal conditions, the constructed Ag@rGO electrochemical sensor has a wide detection range of 0.1-470 μM for L-cysteine, low detection limit of 0.057 μM, and high sensitivity of 215.36 nA M-1 cm-2. In addition, the modified electrode exhibits good anti-interference, reproducibility, and stability.
Keywords: L-cysteine; detection; electrochemical sensor; graphene; nanocomposite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3, 4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk.Anal Chim Acta. 2020 Jan 6;1093:93-105. doi: 10.1016/j.aca.2019.09.043. Epub 2019 Sep 19. Anal Chim Acta. 2020. PMID: 31735219
-
Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water.Biosensors (Basel). 2023 May 21;13(5):563. doi: 10.3390/bios13050563. Biosensors (Basel). 2023. PMID: 37232924 Free PMC article.
-
Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination.Nanomaterials (Basel). 2018 Mar 27;8(4):194. doi: 10.3390/nano8040194. Nanomaterials (Basel). 2018. PMID: 29584682 Free PMC article.
-
Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode.Ultrason Sonochem. 2019 Jul;55:196-206. doi: 10.1016/j.ultsonch.2019.01.038. Epub 2019 Jan 29. Ultrason Sonochem. 2019. PMID: 30878204 Review.
-
Innovative Graphene-Based Nanocomposites for Improvement of Electrochemical Sensors: Synthesis, Characterization, and Applications.Crit Rev Anal Chem. 2024 Apr 24:1-19. doi: 10.1080/10408347.2024.2343854. Online ahead of print. Crit Rev Anal Chem. 2024. PMID: 38656227 Review.
Cited by
-
Composites of Reduced Graphene Oxide Based on Silver Nanoparticles and Their Effect on Breast Cancer Stem Cells.Bioengineering (Basel). 2025 May 11;12(5):508. doi: 10.3390/bioengineering12050508. Bioengineering (Basel). 2025. PMID: 40428127 Free PMC article.
References
-
- Mohammadnavaz A., Beitollahi H., Modiri S. Electro-catalytic determination of L-Cysteine using multi-walled carbon nanotubes-Co3O4 nanocomposite/benzoylferrocene/ionic liquid modified carbon paste electrode. Inorg. Chim. Acta. 2023;548:121340. doi: 10.1016/j.ica.2022.121340. - DOI
-
- Gowtham S.M., Dhivya R., Muthulakshmi L., Sureshkumar S., Ashraf M., Pandi M., Mayandi J., Annaraj J., Sagadevan S. Environmentally benign and biocompatible CuO@Si core-shell nanoparticles: As electrochemical L-cysteine sensor, antibacterial and anti-lung cancer agents. Ceram. Int. 2023;49:10023–10031. doi: 10.1016/j.ceramint.2022.11.182. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

