Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine response in adults with predominantly antibody deficiency
- PMID: 38544577
- PMCID: PMC10965812
- DOI: 10.1016/j.jacig.2024.100234
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine response in adults with predominantly antibody deficiency
Abstract
Background: Patients with predominantly antibody deficiency (PAD) have lower anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibody levels after initial 2-dose SARS-CoV-2 vaccination than healthy controls do; however, the anti-spike antibody responses and neutralization function in patients with PAD following subsequent immunizations remain understudied.
Objective: We sought to characterize anti-spike antibody responses in adults with PAD over the course of 5 SARS-CoV-2 vaccine doses and identify diagnostic and immunophenotypic risk factors for low antibody response.
Methods: We evaluated anti-spike antibody levels in 117 adult patients with PAD and 192 adult healthy controls following a maximum of 5 SARS-CoV-2 immunizations. We assessed neutralization of the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant and analyzed infection outcomes.
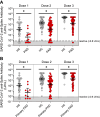
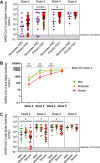
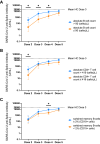
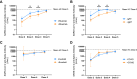
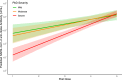
Results: The patients with PAD had significantly lower mean anti-spike antibody levels after 3 SARS-CoV-2 vaccine doses than the healthy controls did (1,439.1 vs 21,890.4 U/mL [P < .0001]). Adults with secondary PAD, severe primary PAD, and high-risk immunophenotypes had lower mean anti-spike antibody levels following vaccine doses 2, 3, and/or 4 but not following vaccine dose 5. Compared with patients with mild and moderate PAD, patients with severe PAD had a higher rate of increase in anti-spike antibody levels over 5 immunizations. A strong positive correlation was observed between anti-spike antibody levels and neutralization of both the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant. Most infections were managed on an outpatient basis.
Conclusions: In all of the patients with PAD, anti-spike antibody levels increased with successive SARS-CoV-2 immunizations and were correlated with neutralization of both the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant. Secondary PAD, severe primary PAD, and high-risk immunophenotypes were correlated with lower mean anti-spike antibody levels following vaccine doses 2 through 4. Patients with severe PAD had the highest rate of increase in anti-spike antibody levels over 5 immunizations. These data suggest a clinical benefit to sequential SARS-CoV-2 immunizations, particularly among high-risk patients with PAD.
Keywords: CD19+ B cells; CD4+ T cells; Omicron BA.5 variant; Predominantly antibody deficiency; SARS-CoV-2; anti-spike antibody; class-switched memory B cells; common variable immunodeficiency; neutralization; rituximab.
© 2024 The Authors.
Figures
References
-
- Boni1lla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186. 205.e1-78. - PubMed
-
- Cheraghi T., Kalantari A., Shabestari M.S., Abolhassani H., Eibel H., Hammarström L. 1st ed. Academic Press; Cambridge, MA: 2021. Inborn errors of immunity.
-
- Durandy A., Kracker S., Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519–533. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

