The alternative coproporphyrinogen III oxidase (CgoN) catalyzes the oxygen-independent conversion of coproporphyrinogen III into coproporphyrin III
- PMID: 38544863
- PMCID: PMC10965808
- DOI: 10.3389/fmicb.2024.1378989
The alternative coproporphyrinogen III oxidase (CgoN) catalyzes the oxygen-independent conversion of coproporphyrinogen III into coproporphyrin III
Abstract
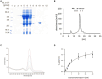
Nature utilizes three distinct pathways to synthesize the essential enzyme cofactor heme. The coproporphyrin III-dependent pathway, predominantly present in Bacillaceae, employs an oxygen-dependent coproporphyrinogen III oxidase (CgoX) that converts coproporphyrinogen III into coproporphyrin III. In this study, we report the bioinformatic-based identification of a gene called ytpQ, encoding a putative oxygen-independent counterpart, which we propose to term CgoN, from Priestia (Bacillus) megaterium. The recombinantly produced, purified, and monomeric YtpQ (CgoN) protein is shown to catalyze the oxygen-independent conversion of coproporphyrinogen III into coproporphyrin III. Minimal non-enzymatic conversion of coproporphyrinogen III was observed under the anaerobic test conditions employed in this study. FAD was identified as a cofactor, and menadione served as an artificial acceptor for the six abstracted electrons, with a KM value of 3.95 μmol/L and a kcat of 0.63 per min for the substrate. The resulting coproporphyrin III, in turn, acts as an effective substrate for the subsequent enzyme of the pathway, the coproporphyrin III ferrochelatase (CpfC). Under aerobic conditions, oxygen directly serves as an electron acceptor, but is replaced by the more efficient action of menadione. An AlphaFold2 model of the enzyme suggests that YtpQ adopts a compact triangular shape consisting of three domains. The N-terminal domain appears to be flexible with respect to the rest of the structure, potentially creating a ligand binding site that opens and closes during the catalytic cycle. A catalytic mechanism similar to the oxygen-independent protoporphyrinogen IX oxidase PgoH1 (HemG), based on the flavin-dependent abstraction of six electrons from coproporphyrinogen III and their potential quinone-dependent transfer to a membrane-localized electron transport chain, is proposed.
Keywords: Bacillaceae; Priestia megaterium; alternative heme biosynthesis; anaerobic metabolism; coproporphyrinogen III oxidase.
Copyright © 2024 Mingers, Barthels, Mass, Acuña, Biedendieck, Cooke, Dailey, Gerdes, Blankenfeldt, Dailey, Warren, Jahn and Jahn.
Conflict of interest statement
TM was employed by the company Pieris Pharmaceuticals GmbH. AC was employed by the company Syngenta UK Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Bali S., Lawrence A. D., Lobo S. A., Saraiva L. M., Golding B. T., Howard M. J., et al. (2011). Molecular hijacking of siroheme for and d1 heme synthesis; elucidation of a new branch of tetrapyrrole synthesis. Proc. Natl. Acad. Sci. U. S. A. 108, 18260–18265. doi: 10.1073/pnas.1108228108, PMID: - DOI - PMC - PubMed
-
- Boynton T. O., Gerddes S., Craven H. H., Neidle E. L., Phillips J. D., Dailey H. A. (2011). Discovery of a gene involved in a third bacterial protoporphyrinogen oxidase activity through comparative genomic analysis and functional complementation. Appl Environ Microbiol. 77, 4795–801. doi: 10.1128/aem.00171-11 - DOI - PMC - PubMed
-
- Dailey T. A., Dailey H. A. (1998). Identification of an FAD superfamily containing protoporphyrinogen oxidases, monoamine oxidases and phytoene desaturase. Expression and characterization of phytoene desaturase of Myxococcus xanthus. J. Biol. Chem. 273, 13658–13662. doi: 10.1074/jbc.273.22.13658, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

