Zinc Nitrate Hexahydrate Pseudobinary Eutectics for Near-Room-Temperature Thermal Energy Storage
- PMID: 38544948
- PMCID: PMC10964232
- DOI: 10.1021/acsaenm.3c00444
Zinc Nitrate Hexahydrate Pseudobinary Eutectics for Near-Room-Temperature Thermal Energy Storage
Abstract
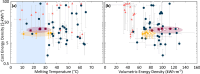
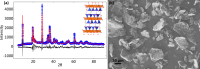
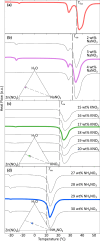
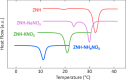
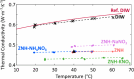
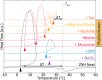
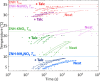
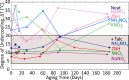
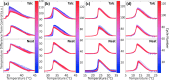
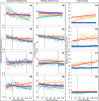
Stoichiometric salt hydrates can be inexpensive and provide higher volumetric energy density relative to other near-room-temperature phase change materials (PCMs), but few salt hydrates exhibit congruent melting behavior between 0 and 30 °C. Eutectic salt hydrates offer a strategy to design bespoke PCMs with tailored application-specific eutectic melting temperatures. However, the general solidification behavior and stability of eutectic salt hydrate systems remain unclear, as metastable solidification in eutectic salt hydrates may introduce opportunities for phase segregation. Here, we present a new family of low-cost zinc-nitrate-hexahydrate-based eutectics: Zn(NO3)2·6(H2O)-NaNO3 (Teu = 32.7 ± 0.3 °C; ΔHeu = 151 ± 6 J·g-1), Zn(NO3)2·6(H2O)-KNO3 (Teu = 22.1 ± 0.3 °C; ΔHeu = 140 ± 6 J·g-1), Zn(NO3)2·6(H2O)-NH4NO3 (Teu = 11.2 ± 0.3 °C; ΔHeu = 137 ± 5 J·g-1). While the tendency to undercool varies greatly between different eutectics in the family, the geologic mineral talc has been identified as an active and stable phase that dramatically reduces undercooling in Zn(NO3)2·6(H2O) and all related eutectics. Zn(NO3)2·6(H2O) and its related eutectics have shown stability for over a hundred thermal cycles in mL scale volumes, suggesting that they are capable of serving as robust and stable media for near-room-temperature thermal energy storage applications in buildings.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Du K.; Calautit J.; Wang Z.; Wu Y.; Liu H. A Review of the Applications of Phase Change Materials in Cooling, Heating and Power Generation in Different Temperature Ranges. Appl. Energy 2018, 220, 242–273. 10.1016/j.apenergy.2018.03.005. - DOI
-
- Henry A.; Prasher R.; Majumdar A. Five Thermal Energy Grand Challenges for Decarbonization. Nat. Energy 2020, 5 (9), 635–637. 10.1038/s41560-020-0675-9. - DOI
-
- Lane G. A. Low Temperature Heat Storage With Phase Change Materials. Int. J. Ambient Energy 1980, 1 (3), 155–168. 10.1080/01430750.1980.9675731. - DOI
-
- Zeng D.; Voigt W. Phase Diagram Calculation of Molten Salt Hydrates Using the Modified BET Equation. CALPHAD 2003, 27, 243–251. 10.1016/j.calphad.2003.09.004. - DOI
-
- Schmit H.; Rathgeber C.; Hennemann P.; Hiebler S. Three–Step Method To Determine the Eutectic Composition of Binary and Ternary Mixtures. J. Therm. Anal. Calorim. 2014, 117 (2), 595–602. 10.1007/s10973-014-3783-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources
