Efficacy and safety of phospholipid nanoparticles (VBI-S) in reversing intractable hypotension in patients with septic shock: a multicentre, open-label, repeated measures, phase 2a clinical pilot trial
- PMID: 38545092
- PMCID: PMC10965406
- DOI: 10.1016/j.eclinm.2024.102430
Efficacy and safety of phospholipid nanoparticles (VBI-S) in reversing intractable hypotension in patients with septic shock: a multicentre, open-label, repeated measures, phase 2a clinical pilot trial
Abstract
Background: Since the 1990's attempts to favorably modulate nitric oxide (NO) have been unsuccessful. We hypothesized that because NO is lipophilic it would preferentially localize into intravascularly infused hydrophobic nanoparticles, thereby reducing its bioavailability and adverse effects without inhibiting its production. We aimed to determine the efficacy and safety of intravenous infusion of a fluid comprised of hydrophobic phospholipid nanoparticles (VBI-S) that reversibly absorb NO in the treatment of hypotension of patients in severe septic shock.
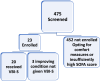
Methods: This is a multicentre, open-label, repeated measures, phase 2a clinical pilot trial done at six hospital centers in the USA. Patients in severe septic shock were enrolled after intravenous fluid therapy had failed to raise mean arterial blood pressure (MAP) to at least the generally accepted level of 65 mmHg, requiring the use of vasopressors. The primary endpoint of this study is the proportion of patients in whom MAP increased by at least 10 mmHg. VBI-S was administered intravenously to patients as boluses of 100 ml, 200 ml, 400 ml, and 800 ml at 999 ml/min until the blood pressure goal was reached after which the infusion was stopped, and the MAP was recorded. All patients who received any volume of VBI-S were included in the primary and safety analysis. The study is registered with ClinicalTrials.gov, NCT04257136.
Findings: Between February 17, 2020 and January 3, 2023, 20 eligible patients were enrolled in the study. In all 20 (100%) patients, the goal of increasing MAP by at least 10 mmHg using VBI-S was achieved (p = 0.0087, effect size = 0.654). Mean VBI-S volume required to meet the primary goal was 561.0 ± 372.3 ml. The goal of lowering vasopressor dose was also achieved (p = 0.0017). Within 48 h or less after VBI-S, there was a statistically significant improvement in oxygenation, serum creatinine, clotting variables, procalcitonin, lactic acid, and the sequential organ failure assessment (SOFA) score. At 24 h and 48 h following administration of VBI-S, 12/15 (80%) and 9/12 (75%) patients developed hyperlipidemia, respectively. No severe adverse events of VBI-S were observed, and there were no treatment-related deaths.
Interpretation: These preliminary findings suggest the safety and efficacy of VBI-S in treating hypotension in patients with septic shock. However, a definitive mortality benefit cannot be demonstrated without a randomized controlled study.
Funding: The Naval Medical Research Command-Naval Advanced Medical Development program via the Medical Technology Enterprise Consortium.
Keywords: Hypotension; Multiple organ dysfunction syndrome; Nitric oxide; Phospholipid nanoparticles; Sepsis.
© 2024 The Author(s).
Conflict of interest statement
Ten US patents and 35 patents in international jurisdictions have been issued to CS for the phospholipid nanoparticle technology. Two patents are pending. One patent application is for the treatment of multiple organ dysfunction syndrome. The other is for the use of phospholipid nanoparticles to reduce inflammation. CS is the principal investigator of the grant from the Naval Medical Research Command. CS is President of Vivacelle Bio, Inc., the company that is the sponsor of the trial and its board chairman. He is also a Vivacelle Bio shareholder. Support for attending scientific meetings is provided to CS by Vivacelle Bio and the UMKC School of Medicine. MK is a contractor for the clinical trial and was paid for his contract work. He is the Senior Vice-President of Vivacelle Bio, Inc., and a Vivacelle Bio Shareholder. HD is CEO of Vivacelle Bio and board member of Vivacelle Bio. He is also a Vivacelle Bio shareholder. Travel expenses are provided by Vivacelle Bio and 2Flo Ventures, an investor in Vivacelle Bio. JR is a member of Vivacelle Bio’s Scientific Advisory Board. Vivacelle Bio gave JR a nitric oxide detector that was used in the manuscript. NH is on the advisory Board of Vivacelle Bio and a Vivacelle Bio shareholder. KT is Chief Medical Officer of Vivacelle Bio and a shareholder of Vivacelle Bio and of Johnson and Johnson. No other authors have a conflict of interest.
Figures
References
-
- Abraham E., Wuderink R., Silverman H., et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor α in patients with sepsis syndrome: a randomized, controlled, double-blind multicenter clinical trial. JAMA. 1995;273(12):934–941. - PubMed
-
- Opal S.M., Fisher C.J., Jr., Dhainaut J.F., et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. - PubMed
-
- FDA safety announcement: FDA drug safety communication: voluntary market withdrawal of Xigris [drotrecogin alfa (activated)] due to failure to show a survival benefit 10-25-2011. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c...
-
- Blazoski C., McDermott L., Yang Q., Hirose H. Outcomes of extracorporeal membrane oxygenation in patients with blood culture positive septic. Cardiovasc Dis. 2020;158(4)
Associated data
LinkOut - more resources
Full Text Sources
Medical