Risk factors for graft-versus-host-disease after donor lymphocyte infusion following T-cell depleted allogeneic stem cell transplantation
- PMID: 38545096
- PMCID: PMC10966113
- DOI: 10.3389/fimmu.2024.1335341
Risk factors for graft-versus-host-disease after donor lymphocyte infusion following T-cell depleted allogeneic stem cell transplantation
Abstract
Introduction: Unmodified donor lymphocyte infusions (DLI) after allogeneic stem cell transplantation (alloSCT) can boost the beneficial Graft-versus-Leukemia (GvL) effect but may also induce severe Graft-versus-Host-Disease (GvHD). To improve the balance between GvL and GvHD, it is crucial to identify factors that influence the alloreactivity of DLI.
Methods: We investigated the effects of the presence of patient-derived antigen-presenting cells at time of DLI as estimated by the bone marrow (BM) chimerism status, lymphopenia as measured by the absolute lymphocyte count (ALC) at time of DLI, and the presence of a viral infection (de novo or reactivation) close to DLI on the risk of GvHD after DLI. The cohort consisted of patients with acute leukemia or myelodysplastic syndrome who prophylactically or pre-emptively received DLI as standard care after alemtuzumab-based alloSCT. In patients at high risk for relapse, DLI was administered at 3 months after alloSCT (n=88) with a dose of 0.3x106 or 0.15x106 T cells/kg in case of a related or unrelated donor, respectively. All other patients (n=76) received 3x106 or 1.5x106 T cells/kg, respectively, at 6 months after alloSCT.
Results: For both DLIs, patients with reduced-intensity conditioning and an unrelated donor had the highest risk of GvHD. For DLI given at three months, viral infection within 1 week before and 2 weeks after DLI was an additional significant risk factor (hazard ratio (HR) 3.66 compared to no viral infection) for GvHD. At six months after alloSCT, viral infections were rare and not associated with GvHD. In contrast, mixed BM chimerism (HR 3.63 for ≥5% mixed chimerism compared to full donor) was an important risk factor for GvHD after DLI given at six months after alloSCT. ALC of <1000x106/l showed a trend for association with GvHD after this DLI (HR 2.05 compared to ≥1000x106/l, 95% confidence interval 0.94-4.45). Furthermore, the data suggested that the presence of a viral infection close to the DLI at three months or ≥5% mixed chimerism at time of the DLI at six months correlated with the severity of GvHD, thereby increasing their negative impact on the current GvHD-relapse-free survival.
Conclusion: These data demonstrate that the risk factors for GvHD after DLI depend on the setting of the DLI.
Keywords: acute lymphoblastic leukemia; acute myeloid leukemia; allogeneic stem cell transplantation; donor lymphocyte infusion; graft-versus-host-disease; multi-state modelling; myelodysplastic syndrome.
Copyright © 2024 Koster, von dem Borne, van Balen, Marijt, Tjon, Snijders, van Lammeren, Veelken, Falkenburg, Halkes and de Wreede.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
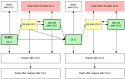
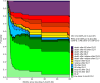


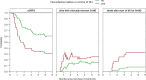
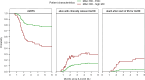
References
-
- Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. . Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. (2017) 35:4003–11. doi: 10.1200/jco.2017.75.8177 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

