The effects of vitamin D supplementation on inflammatory biomarkers in patients with asthma: a systematic review and meta-analysis of randomized controlled trials
- PMID: 38545098
- PMCID: PMC10965564
- DOI: 10.3389/fimmu.2024.1335968
The effects of vitamin D supplementation on inflammatory biomarkers in patients with asthma: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: While the association between vitamin D and several inflammatory biomarkers in asthma patients has been extensively reported, it remains unclear whether supplementation modifies these biomarkers. This review aims to evaluate the impact of vitamin D supplementation on inflammatory biomarkers measured in vivo in individuals with asthma.
Methods: We conducted a systematic review of randomized controlled trials (RCTs) published until November 2022 in six electronic databases evaluating the impact of vitamin D supplementation (any dose, form, administration route, frequency, or duration) compared to placebo in children or adults. The two co-primary outcomes were serum IgE and blood eosinophils reported at the endpoint. Secondary outcomes included other markers of type 2 inflammation (e.g., sputum eosinophils, fractional exhaled nitric oxide, etc.), anti-inflammatory biomarkers (e.g., interleukin (IL)-10, etc.), markers of non-type 2 inflammation (e.g., high-sensitivity C-reactive protein, etc.), and non-specific biomarkers (e.g., macrophages, etc.). Data were aggregated using fixed or random effect models.
Results: Thirteen RCTs (5 in adults, 5 in pediatric patients, and 3 in mixed age groups) testing doses of vitamin D supplementation ranging from 800 to 400,000 IU over periods of 6 weeks to 12 months were included. Eight studies provided data on serum IgE and four on blood eosinophils. As secondary outcomes, three studies reported on sputum eosinophils, four on FeNO, five on serum IL-10, and two on airway IL-10. Compared to placebo, vitamin D supplementation had no significant effect on serum IgE (Mean difference [MD] [95% CI]: 0.06 [-0.13, 0.26] IU/mL), blood eosinophils (MD [95% CI]: - 0.02 [-0.11, 0.07] 103/μL), or FeNO (MD [95% CI]: -4.10 [-10.95, 2.75] ppb) at the endpoint. However, the vitamin D supplementation group showed higher serum IL-10 levels compared to placebo (MD [95% CI]: 18.85 [1.11, 36.59] pg/ml) at the endpoint. Although data could not be aggregated, narrative synthesis suggested no significant effect of supplementation on sputum eosinophils and IL-10 in both sputum and exhaled breath condensate, at the endpoint.
Conclusion: Vitamin D supplementation in individuals with asthma was not associated with lower inflammatory biomarkers related to type 2 inflammation. However, it was significantly associated with higher serum IL-10 compared to placebo.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022365666.
Keywords: asthma; biomarkers; inflammation; meta-analysis; systematic review; vitamin D.
Copyright © 2024 El Abd, Dasari, Dodin, Trottier and Ducharme.
Conflict of interest statement
FMD has received unrestricted research funds from Jamieson and GSK, as well as investigator initiated research funds from Covis Pharma, Banque Scotia Foundation, GlaxoSmithKline, and MEDteq in partnership with Thorasys Inc. FMD has received an honorarium for consultancy work from INESSS, Astra Zeneca, Covis Pharma, Ontario Lung Association, Sanofi, and Teva. FMD has also been on the advisory board for Sanofi and has received honoraria as an invited speaker from the Association des Médecins omnipraticiens du Richelieu Saint-Laurent, Covis Pharma, Réseau québécois d’éducation en santé respiratoire (RQESR), Sanofi-Regeneron, Thorasys Inc., and Trudell Medical International. HT reported receiving occasional lecture fees from Merck and unrestricted grants form ViiV Healthcare. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



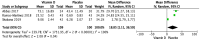
References
-
- Brehm J, Schuemann B, Fuhlbrigge A, Hollis B, Strunk R, Zeiger R, et al. . Childhood Asthma Management Program Research Group Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. (2010) 126:52–8. doi: 10.1016/j.jaci.2010.03.043 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

