Proteome-wide assessment of human interactome as a source of capturing domain-motif and domain-domain interactions
- PMID: 38545252
- PMCID: PMC10964934
- DOI: 10.1002/ccs3.12014
Proteome-wide assessment of human interactome as a source of capturing domain-motif and domain-domain interactions
Abstract
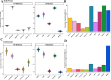
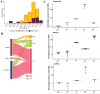
Protein-protein interactions (PPIs) play a crucial role in various biological processes by establishing domain-motif (DMI) and domain-domain interactions (DDIs). While the existence of real DMIs/DDIs is generally assumed, it is rarely tested; therefore, this study extensively compared high-throughput methods and public PPI repositories as sources for DMI and DDI prediction based on the assumption that the human interactome provides sufficient data for the reliable identification of DMIs and DDIs. Different datasets from leading high-throughput methods (Yeast two-hybrid [Y2H], Affinity Purification coupled Mass Spectrometry [AP-MS], and Co-fractionation-coupled Mass Spectrometry) were assessed for their ability to capture DMIs and DDIs using known DMI/DDI information. High-throughput methods were not notably worse than PPI databases and, in some cases, appeared better. In conclusion, all PPI datasets demonstrated significant enrichment in DMIs and DDIs (p-value <0.001), establishing Y2H and AP-MS as reliable methods for predicting these interactions. This study provides valuable insights for biologists in selecting appropriate methods for predicting DMIs, ultimately aiding in SLiM discovery.
Keywords: DDIs; DMIs; PPIs; SLiMs; high‐throughput methods; proteome.
© 2024 The Authors. Journal of Cell Communication and Signaling published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Peng, Xiaoqing. , Jianxin Wang., Wei Peng., Fang‐Xiang Wu, and Yi Pan. 2017. “Protein‐Protein Interactions: Detection, Reliability Assessment and Applications.” Briefings in Bioinformatics 18(5): 798–819. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

