Human extracellular matrix (ECM)-like collagen and its bioactivity
- PMID: 38545260
- PMCID: PMC10965421
- DOI: 10.1093/rb/rbae008
Human extracellular matrix (ECM)-like collagen and its bioactivity
Abstract
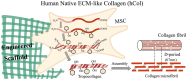
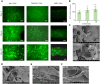
Collagen, the most abundant structural protein in the human extracellular matrix (ECM), provides essential support for tissues and guides tissue development. Despite its widespread use in tissue engineering, there remains uncertainty regarding the optimal selection of collagen sources. Animal-derived sources pose challenges such as immunogenicity, while the recombinant system is hindered by diminished bioactivity. In this study, we hypothesized that human ECM-like collagen (hCol) could offer an alternative for tissue engineering. In this study, a facile platform was provided for generating hCol derived from mesenchymal stem cells with a hierarchical structure and biochemical properties resembling native collagen. Our results further demonstrated that hCol could facilitate basal biological behaviors of human adipose-derived stem cells, including viability, proliferation, migration and adipocyte-like phenotype. Additionally, it could promote cutaneous wound closure. Due to its high similarity to native collagen and good bioactivity, hCol holds promise as a prospective candidate for in vitro and in vivo applications in tissue engineering.
Keywords: cell-derived matrix; extracellular matrix; hierarchical structure; native-like collagen.
© The Author(s) 2024. Published by Oxford University Press.
Figures





Similar articles
-
Molecular, biochemical and functional analysis of a novel and developmentally important fibrillar collagen (Hcol-I) in hydra.Development. 2000 Nov;127(21):4669-80. doi: 10.1242/dev.127.21.4669. Development. 2000. PMID: 11023869
-
Intact vitreous humor as a potential extracellular matrix hydrogel for cartilage tissue engineering applications.Acta Biomater. 2019 Feb;85:117-130. doi: 10.1016/j.actbio.2018.12.022. Epub 2018 Dec 18. Acta Biomater. 2019. PMID: 30572166
-
Human Adipose-Derived Mesenchymal Stromal/Stem Cell Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like Extracellular Matrix Pattern.Tissue Eng Part A. 2020 Aug;26(15-16):915-926. doi: 10.1089/ten.TEA.2019.0206. Epub 2020 Apr 9. Tissue Eng Part A. 2020. PMID: 32070231
-
Collagenous Extracellular Matrix Biomaterials for Tissue Engineering: Lessons from the Common Sea Urchin Tissue.Int J Mol Sci. 2017 Apr 25;18(5):901. doi: 10.3390/ijms18050901. Int J Mol Sci. 2017. PMID: 28441344 Free PMC article. Review.
-
Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives.Polymers (Basel). 2016 Feb 4;8(2):42. doi: 10.3390/polym8020042. Polymers (Basel). 2016. PMID: 30979136 Free PMC article. Review.
Cited by
-
Rational design matrix materials for organoid development and application in biomedicine.Regen Biomater. 2025 May 14;12:rbaf038. doi: 10.1093/rb/rbaf038. eCollection 2025. Regen Biomater. 2025. PMID: 40556786 Free PMC article. Review.
-
Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review.Int J Mol Sci. 2024 Nov 30;25(23):12911. doi: 10.3390/ijms252312911. Int J Mol Sci. 2024. PMID: 39684619 Free PMC article. Review.
-
Evaluation of Biotechnological Active Peptides Secreted by Saccharomyces cerevisiae with Potential Skin Benefits.Antibiotics (Basel). 2024 Sep 13;13(9):881. doi: 10.3390/antibiotics13090881. Antibiotics (Basel). 2024. PMID: 39335054 Free PMC article.
-
Engineering of tissue in microphysiological systems demonstrated by modelling skeletal muscle.Regen Biomater. 2025 Jun 16;12:rbaf059. doi: 10.1093/rb/rbaf059. eCollection 2025. Regen Biomater. 2025. PMID: 40717794 Free PMC article. Review.
-
Puerarin: a hepatoprotective drug from bench to bedside.Chin Med. 2024 Oct 8;19(1):139. doi: 10.1186/s13020-024-01011-y. Chin Med. 2024. PMID: 39380120 Free PMC article. Review.
References
-
- Heino J. The collagen family members as cell adhesion proteins. Bioessays 2007;29:1001–10. - PubMed
-
- Hadjipanayi E, Mudera V, Brown RA.. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton 2009;66:121–8. - PubMed
-
- Glowacki J, Mizuno S.. Collagen scaffolds for tissue engineering. Biopolymers 2008;89:338–44. - PubMed
LinkOut - more resources
Full Text Sources

