Acute Rift Valley fever virus infection induces inflammatory cytokines and cell death in ex vivo rat brain slice culture
- PMID: 38546100
- PMCID: PMC10995633
- DOI: 10.1099/jgv.0.001970
Acute Rift Valley fever virus infection induces inflammatory cytokines and cell death in ex vivo rat brain slice culture
Abstract
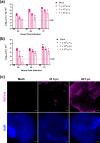
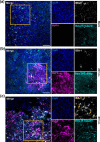
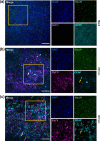
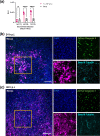
Rift Valley fever virus (RVFV) is an emerging arboviral disease with pandemic potential. While infection is often self-limiting, a subset of individuals may develop late-onset encephalitis, accounting for up to 20 % of severe cases. Importantly, individuals displaying neurologic disease have up to a 53 % case fatality rate, yet the neuropathogenesis of RVFV infection remains understudied. In this study, we evaluated whether ex vivo postnatal rat brain slice cultures (BSCs) could be used to evaluate RVFV infection in the central nervous system. BSCs mounted an inflammatory response after slicing, which resolved over time, and they were viable in culture for at least 12 days. Infection of rat BSCs with pathogenic RVFV strain ZH501 induced tissue damage and apoptosis over 48 h. Viral replication in BSCs reached up to 1×107 p.f.u. equivalents/ml, depending on inoculation dose. Confocal immunofluorescent microscopy of cleared slices confirmed direct infection of neurons as well as activation of microglia and astrocytes. Further, RVFV-infected rat BSCs produced antiviral cytokines and chemokines, including MCP-1 and GRO/KC. This study demonstrates that rat BSCs support replication of RVFV for ex vivo studies of neuropathogenesis. This allows for continued and complementary investigation into RVFV infection in an ex vivo postnatal brain slice culture format.
Keywords: Rift Valley fever virus; brain slice culture; bunyavirus; ex vivo model; neurotropic virus; viral encephalitis.
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

