Human cytomegalovirus seropositivity and its influence on oral rotavirus vaccine immunogenicity: a specific concern for HIV-exposed-uninfected infants
- PMID: 38546123
- PMCID: PMC11188542
- DOI: 10.1093/cei/uxae029
Human cytomegalovirus seropositivity and its influence on oral rotavirus vaccine immunogenicity: a specific concern for HIV-exposed-uninfected infants
Abstract
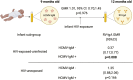
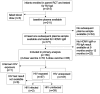
Oral rotavirus vaccines demonstrate diminished immunogenicity in low-income settings where human cytomegalovirus infection is acquired early in childhood and modulates immunity. We hypothesized that human cytomegalovirus infection around the time of vaccination may influence immunogenicity. We measured plasma human cytomegalovirus-specific immunoglobulin M antibodies in rotavirus vaccinated infants from 6 weeks to 12 months old and compared rotavirus immunoglobulin A antibody titers between human cytomegalovirus seropositive and seronegative infants. There was no evidence of an association between human cytomegalovirus serostatus at 9 months and rotavirus-specific antibody titers at 12 months (geometric mean ratio 1.01, 95% CI: 0.70, 1.45; P = 0.976) or fold-increase in RV-IgA titer between 9 and 12 months (risk ratio 0.999, 95%CI: 0.66, 1.52; P = 0.995) overall. However, HIV-exposed-uninfected infants who were seropositive for human cytomegalovirus at 9 months old had a 63% reduction in rotavirus antibody geometric mean titers at 12 months compared to HIV-exposed-uninfected infants who were seronegative for human cytomegalovirus (geometric mean ratio 0.37, 95% CI: 0.17, 0.77; P = 0.008). While the broader implications of human cytomegalovirus infections on oral rotavirus vaccine response might be limited in the general infant population, the potential impact in the HIV-exposed-uninfected infants cannot be overlooked. This study highlights the complexity of immunological responses and the need for targeted interventions to ensure oral rotavirus vaccine efficacy, especially in vulnerable subpopulations.
Keywords: Zambia; antibody; human cytomegalovirus; rotavirus; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Mwenda JM, Hallowell BD, Parashar U, Shaba K, Biey JN, Weldegebriel GG, et al.. Impact of rotavirus vaccine introduction on rotavirus hospitalizations among children under 5 years of age—World Health Organization African Region, 2008–2018. Clin Infect Dis 2021, 73, 1605–8. doi:10.1093/cid/ciab520 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

