Safety, Efficacy, and Biological Data of T-Cell-Enabling Oncolytic Adenovirus TILT-123 in Advanced Solid Cancers from the TUNIMO Monotherapy Phase I Trial
- PMID: 38546220
- PMCID: PMC11369615
- DOI: 10.1158/1078-0432.CCR-23-3874
Safety, Efficacy, and Biological Data of T-Cell-Enabling Oncolytic Adenovirus TILT-123 in Advanced Solid Cancers from the TUNIMO Monotherapy Phase I Trial
Abstract
Purpose: TILT-123 (igrelimogene litadenorepvec) is an oncolytic adenovirus armed with TNFa and IL2, designed to induce T-cell infiltration and cytotoxicity in solid tumors.
Patients and methods: TUNIMO (NCT04695327) was a single-arm, multicenter phase I dose-escalation trial designed to assess the safety of TILT-123 in advanced solid cancers refractory to standard therapy. Patients received intravenous and intratumoral TILT-123. The primary endpoint was safety by adverse events (AE), laboratory values, vital signs, and electrocardiograms. Secondary endpoints included tumor response, pharmacokinetics, and predictive biomarkers.
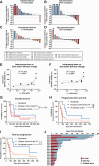
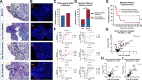
Results: Twenty patients were enrolled, with a median age of 58 years. Most prevalent cancer types included sarcomas (35%), melanomas (15%) and ovarian cancers (15%). No dose-limiting toxicities were observed. The most frequent treatment-related AEs included fever (16.7%), chills (13.0%), and fatigue (9.3%). Ten patients were evaluable for response on day 78 with RECIST 1.1, iRECIST or PET-based evaluation. The disease control rate by PET was 6/10 (60% of evaluable patients) and 2/10 by RECIST 1.1 and iRECIST(20%of evaluable patients). Tumor size reductions occurred in both injected and non-injected lesions. TILT-123 was detected in injected and non-injected tumors, and virus was observed in blood after intravenous and intratumoral injections. Treatment resulted in reduction of lymphocytes in blood, with concurrent lymphocyte increases in tumors, findings compatible with trafficking.
Conclusions: TILT-123 was safe and able to produce antitumor effects in local and distant lesions in heavily pre-treated patients. Good tolerability of TILT-123 facilitates combination studies, several of which are ongoing (NCT04217473, NCT05271318, NCT05222932, and NCT06125197). See related commentary by Silva-Pilipich and Smerdou, p. 3649.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S.A. Pakola reports grants from Helsinki University Hospital Research Funds, Cancer Foundation Finland, Jane and Aatos Erkko Foundation, Red Cross Blood Service, and Sigrid Juselius Finland, other support from TILT Biotherapeutics Oy, and grants from the European Commission during the conduct of the study. K.J. Peltola reports other support from TILT Therapeutics during the conduct of the study and from Faron Pharmaceuticals outside the submitted work, as well as personal fees from BMS, MSD, IPSEN, Roche, Bayer, and Novartis. J.H.A. Clubb reports being employed and a shareholder at TILT Biotherapeutics. L. Haybout reports other support from TILT Biotherapeutics during the conduct of the study. T. Alanko reports other support from TILT Biotherapeutics during the conduct of the study; personal fees and non-financial support from AstraZeneca and MSD; personal fees and other support from Bristol Myers Squibb, Roche, and Incyte; personal fees, non-financial support, and other support from Pfizer; personal fees from Servier and Nordic Drugs; other support from AbbVie, Bayer, Boehringer Ingelheim, Lilly, Debiopharm Group, GlaxoSmithKline, and TILT Biotherapeutics; and non-financial support from Merck outside the submitted work. A. Hemmes reports other support from TILT Biotherapeutics during the conduct of the study. T. Pellinen reports grants from Roche Glycart AG outside the submitted work. D.C. Quixabeira reports personal fees from TILT Biotherapeutics during the conduct of the study and outside the submitted work. C. Kistler reports other support from TILT Biotherapeutics and from TILT Biotherapeutics during the conduct of the study. S. Sorsa reports grants from the European Innovation Council—European Commission and personal fees from TILT Biotherapeutics during the conduct of the study. R. Havunen reports personal fees from TILT Biotherapeutics and other support from TILT Biotherapeutics during the conduct of the study. J.M. Santos reports personal fees, non-financial support, and other support from TILT Biotherapeutics; grants from the European Innovation Council and Business Finland during the conduct of the study; and personal fees, non-financial support, and other support from TILT Biotherapeutics outside the submitted work. V. Cervera-Carrascon reports personal fees from TILT Biotherapeutics during the conduct of the study and outside the submitted work. A. Hemminki reports grants from Helsinki University Hospital Research funds, Cancer Foundation Finland, Jane and Aatos Erkko Foundation, Red Cross Blood Service, and Sigrid Juselius Finland; other support from TILT Biotherapeutics Oy; grants from the European Commission during the conduct of the study; personal fees and other support from TILT Biotherapeutics Oy and Aeruginosa Oy; and other support from Circio Holdings ASA outside the submitted work; in addition, A. Hemminki reports patents for Enhanced adoptive cell therapy licensed to TILT Biotherapeutics Oy, Oncolytic adenoviruses coding for BI-specific antibodies and methods and uses related thereto licensed to TILT Biotherapeutics Oy, Oncolytic adenovirus and checkpoint inhibitor combination therapy pending to TILT Biotherapeutics Oy, Oncolytic virus vector coding for variant IL2 (vIL-2) polypeptide pending to TILT Biotherapeutics Oy, and Oncolytic adenovirus combination therapy pending to TILT Biotherapeutics Oy. No disclosures were reported by the other authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

