BCMA x CD3 T-cell engager in a patient with pentarefractory multiple myeloma and HIV: a clinical and immunological report
- PMID: 38546668
- PMCID: PMC11367224
- DOI: 10.3324/haematol.2023.284917
BCMA x CD3 T-cell engager in a patient with pentarefractory multiple myeloma and HIV: a clinical and immunological report
Figures


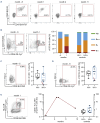
References
-
- Moreau P, Kumar SK, San Miguel J, et al. . Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e105-e118. - PubMed
-
- Moreau P, van de Donk NWCJ, Delforge M, et al. . Comparative efficacy of teclistamab versus current treatments in real-world clinical practice in the Prospective LocoMMotion Study in patients with triple-class-exposed relapsed and/or refractory multiple myeloma. Adv Ther. 2023;40(5):2412-2425. - PMC - PubMed
-
- Hattenhauer ST, Mispelbaum R, Hentrich M, Boesecke C, Monin MB. Enabling CAR T-cell therapies for HIV-positive lymphoma patients - A call for action. HIV Med. 2023;24(9):957-964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

