Maintenance of Investigator's Static Global Assessment Response with Once-Daily Crisaborole in Participants with Mild to Moderate Atopic Dermatitis
- PMID: 38546803
- PMCID: PMC11052956
- DOI: 10.1007/s13555-024-01129-9
Maintenance of Investigator's Static Global Assessment Response with Once-Daily Crisaborole in Participants with Mild to Moderate Atopic Dermatitis
Abstract
Introduction: Treatments for atopic dermatitis (AD) often fail to achieve lasting disease control. In the CrisADe CONTROL phase III study (ClinicalTrials.gov: NCT04040192), participants aged ≥ 3 months with mild to moderate AD treated with once-daily (QD) crisaborole, following initial treatment success with crisaborole twice daily (BID), had longer periods of flare-free maintenance, a higher number of flare-free days, and a lower number of flares compared with those who received vehicle. The study was an exploratory analysis of data on the maintenance of response per Investigator's Static Global Assessment (ISGA; ISGA score of 0 [clear] or 1 [almost clear]) during the CrisADe CONTROL study through week 52.
Methods: Exploratory endpoints were the time to ISGA response during the open-label run-in period, and the maintenance of ISGA response and the severity and duration of flares during the double-blind maintenance period. Outcomes were stratified by age (participants aged 3 months to < 12 years and ≥ 12 years) and duration of crisaborole BID treatment (< 4 weeks or ≥ 4 weeks) during the open-label run-in period.
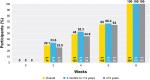
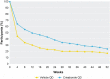
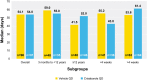
Results: During the open-label run-in period, the median time to ISGA response was 41.5 days. From week 4 to week 52 of the double-blind maintenance period, the proportion of participants who maintained ISGA response was greater with crisaborole versus vehicle, and this difference was statistically significant up to week 36 (P < 0.05). Duration of flare periods during the maintenance period were 54.1 and 54.0 days for the vehicle and crisaborole-treated groups, respectively. Numerically fewer crisaborole-treated participants experienced a flare with an ISGA score of ≥ 2 compared with vehicle-treated participants (64.8% vs. 74.4%, respectively). Findings were comparable across most subgroups.
Conclusions: Adult and pediatric participants with mild to moderate AD at baseline who had achieved responder criteria (treatment success) with crisaborole BID during the run-in period maintained response per ISGA with crisaborole QD during the double-blind maintenance period through week 52.
Trial registration: ClinicalTrials.gov: NCT04040192.
Keywords: Adults; Anti-inflammatory agents; Atopic dermatitis; Crisaborole; Disease control; Flare; Investigator’s Static Global Assessment; Maintenance; Pediatrics.
Plain language summary
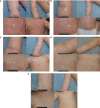
Atopic dermatitis is a skin disease that causes itchy, red, and dry patches of skin that can affect a person for a long time. Current treatments for atopic dermatitis often fail to keep the symptoms under control. Some creams and ointments applied to the skin (known as topical treatments) can ease the discomfort of atopic dermatitis. Crisaborole is a steroid-free ointment that has been shown to improve symptoms of atopic dermatitis in clinical studies. In a study called the CrisADe CONTROL trial, crisaborole was tested to see if it can keep atopic dermatitis symptoms under control. People who participated in the study were aged 3 months and older and they had mild-to-moderate atopic dermatitis. Participants were asked to use crisaborole on their itchy, red, and dry skin twice daily for 8 weeks. Patients were called “responders” if their symptoms became nearly clear or completely clear based on a doctor’s assessment called the Investigator’s Static Global Assessment, which rates atopic dermatitis between clear to severe. Some responders were asked to use crisaborole once daily for 52 weeks and another group of responders was asked to use a control (an ointment with no medicine) once daily for 52 weeks. Investigators looked at how long the skin remained nearly clear or completely clear during the 52 weeks. Results of this study showed that after initial treatment success with crisaborole twice daily, adult and pediatric participants who had mild-to-moderate atopic dermatitis were able to keep their skin nearly clear or completely clear with crisaborole once daily.
© 2024. The Author(s).
Conflict of interest statement
Lawrence F. Eichenfield has served as a scientific advisor, consultant, and/or clinical trial investigator for Pfizer Inc., AbbVie, Amgen, Aslan Pharmaceuticals, Castle Biosciences, Dermavant, Eli Lilly and Company, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Regeneron Pharmaceuticals, and Sanofi-Genzyme. Linda F. Stein-Gold has received grants from Pfizer Inc., Incyte, and LEO Pharma and has received payment for lectures from Pfizer Inc. and LEO Pharma. Charles Lynde has been a speaker, consultant, and/or principal investigator for Pfizer Inc., AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Eli Lilly and Company, Fresnius Kabi, Galderma, GSK, InCyte, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche-Posay, LEO Pharma, L’Oreal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Regeneron, Roche, Sanofi-Genzyme, Sandoz, Sentrex, SunPharma, TEVA, Tribute, UCB, Valeant, Viatris, and Volo Health, and has served as a principal investigator for Acelyrin, Akros, Altius, Avillion, Celltrion, Concert, Devonian, Evelo, and MoonLake. Lyn Guenther has received consulting fees/honoraria from Pfizer Inc., Aralez, Bausch Health, BMS, Cipher, Eli Lilly and Company, GSK, Incyte, Janssen, Johnson and Johnson, La Roche-Posay, LEO Pharma, Merck, Miravo, Novartis, and UCB; has been a member of a Speaker’s Bureau/advisory board member for Pfizer Inc., Abbvie, Actilion, Amgen, Altana, Aralez, Aslan, Bausch Health, Eli Lilly and Company, Galderma, Janssen, Johnson and Johnson, La Roche-Posay, LEO Pharma, Novartis, and Sanofi Aventis, UCB; and has received research grants from Pfizer Inc., Abbvie, Actilion, Amgen, Bausch Health, BMS, Boehringer Ingelheim, Celgene, Cipher, Eli Lilly and Company, Galderma, Innovaderm, Janssen, La Roche-Posay, LEO Pharma, Merck, Novartis, and UCB. Shoshana Greenberger served as an investigator for Pfizer Inc., AbbVie, Amgen, Eli Lilly and Company, and, Novartis; has served on a Speaker’s Bureau/advisory board for Pfizer Inc., AbbVie, Sanofi, Pfizer, Padagis, and Dexcel Pharma; has provided investigator services for Pfizer Inc., AbbVie, Amgen, and Eli Lilly and Company; and has received research grants from Pfizer. Chia-Yu Chu has served as an investigator for Pfizer Inc., AbbVie, Amgen, Dermira, Eli Lilly and Company, Janssen, Novartis, Oneness Biotech, Regeneron, Roche, and Sanofi; has served as a consultant for Pfizer Inc., AbbVie, Eli Lilly and Company, Janssen, Novartis, Roche, and Sanofi; has served as a speaker for Pfizer Inc., AbbVie, Eli Lilly and Company, Janssen, Mylan, Novartis, Roche, Sanofi, and Viatris; and has served on the advisory boards of Pfizer Inc., AbbVie, Amgen, Eli Lilly and Company, Janssen, Mylan, Roche, Sanofi, and Viatris. Bonnie Vlahos, Paul Sanders, Amy Cha, Juliana M. Canosa, and Zara Ghodsi are employees and shareholders of Pfizer Inc.
Figures