Integration of a fasting-mimicking diet programme in primary care for type 2 diabetes reduces the need for medication and improves glycaemic control: a 12-month randomised controlled trial
- PMID: 38546821
- PMCID: PMC11153305
- DOI: 10.1007/s00125-024-06137-0
Integration of a fasting-mimicking diet programme in primary care for type 2 diabetes reduces the need for medication and improves glycaemic control: a 12-month randomised controlled trial
Abstract
Aims/hypothesis: The aim of this study was to evaluate the impact on metabolic control of periodic use of a 5-day fasting-mimicking diet (FMD) programme as an adjunct to usual care in people with type 2 diabetes under regular primary care surveillance.
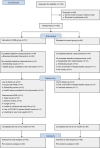
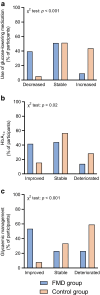
Methods: In this randomised, controlled, assessor-blinded trial, people with type 2 diabetes using metformin as the only glucose-lowering drug and/or diet for glycaemic control were randomised to receive 5-day cycles of an FMD monthly as an adjunct to regular care by their general practitioner or to receive regular care only. The primary outcomes were changes in glucose-lowering medication (as reflected by the medication effect score) and HbA1c levels after 12 months. Moreover, changes in use of glucose-lowering medication and/or HbA1c levels in individual participants were combined to yield a clinically relevant outcome measure ('glycaemic management'), which was categorised as improved, stable or deteriorated after 1 year of follow-up. Several secondary outcome measures were also examined, including changes in body weight.
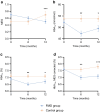
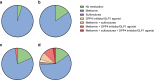
Results: One hundred individuals with type 2 diabetes, age 18-75 years, BMI ≥27 kg/m2, were randomised to the FMD group (n=51) or the control group (n=49). Eight FMD participants and ten control participants were lost to follow-up. Intention-to-treat analyses, using linear mixed models, revealed adjusted estimated treatment effects for the medication effect score (-0.3; 95% CI -0.4, -0.2; p<0.001), HbA1c (-3.2 mmol/mol; 95% CI -6.2, -0.2 and -0.3%; 95% CI -0.6, -0.0; p=0.04) and body weight (-3.6 kg; 95% CI -5.2, -2.1; p<0.001) at 12 months. Glycaemic management improved in 53% of participants using FMD vs 8% of control participants, remained stable in 23% vs 33%, and deteriorated in 23% vs 59% (p<0.001).
Conclusions/interpretation: Integration of a monthly FMD programme in regular primary care for people with type 2 diabetes who use metformin as the only glucose-lowering drug and/or diet for glycaemic control reduces the need for glucose-lowering medication, improves HbA1c despite the reduction in medication use, and appears to be safe in routine clinical practice.
Trial registration: ClinicalTrials.gov NCT03811587 FUNDING: The project was co-funded by Health~Holland, Top Sector Life Sciences & Health, the Dutch Diabetes Foundation and L-Nutra.
Keywords: Diet; Fasting-mimicking diet; Glucose-lowering medication; HbA1c, Lifestyle; Primary care; Randomised controlled trial; Therapy; Type 2 diabetes.
© 2024. The Author(s).
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

