Brainstem depolarization-induced lethal apnea associated with gain-of-function SCN1AL263V is prevented by sodium channel blockade
- PMID: 38547067
- PMCID: PMC10998578
- DOI: 10.1073/pnas.2309000121
Brainstem depolarization-induced lethal apnea associated with gain-of-function SCN1AL263V is prevented by sodium channel blockade
Abstract
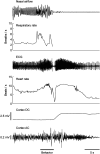
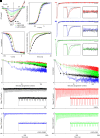
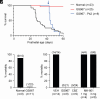
Apneic events are frightening but largely benign events that often occur in infants. Here, we report apparent life-threatening apneic events in an infant with the homozygous SCN1AL263V missense mutation, which causes familial hemiplegic migraine type 3 in heterozygous family members, in the absence of epilepsy. Observations consistent with the events in the infant were made in an Scn1aL263V knock-in mouse model, in which apnea was preceded by a large brainstem DC-shift, indicative of profound brainstem depolarization. The L263V mutation caused gain of NaV1.1 function effects in transfected HEK293 cells. Sodium channel blockade mitigated the gain-of-function characteristics, rescued lethal apnea in Scn1aL263V mice, and decreased the frequency of severe apneic events in the patient. Hence, this study shows that SCN1AL263V can cause life-threatening apneic events, which in a mouse model were caused by profound brainstem depolarization. In addition to being potentially relevant to sudden infant death syndrome pathophysiology, these data indicate that sodium channel blockers may be considered therapeutic for apneic events in patients with these and other gain-of-function SCN1A mutations.
Keywords: apnea; brainstem; sodium channel; sudden death.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- DiMario F. J. Jr., Breath-holding spells in childhood. Am. J. Dis. Child. 146, 125–131 (1992). - PubMed
-
- Kahn A., et al. , Sleep and cardiorespiratory characteristics of infant victims of sudden death: A prospective case-control study. Sleep 15, 287–292 (1992). - PubMed
-
- Thach B. T., Potential central nervous system involvement in sudden unexpected infant deaths and the sudden infant death syndrome. Compr. Physiol. 5, 1061–1068 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

