Molecular mechanism of dynein-dynactin complex assembly by LIS1
- PMID: 38547289
- PMCID: PMC7615804
- DOI: 10.1126/science.adk8544
Molecular mechanism of dynein-dynactin complex assembly by LIS1
Abstract
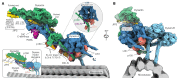
Cytoplasmic dynein is a microtubule motor vital for cellular organization and division. It functions as a ~4-megadalton complex containing its cofactor dynactin and a cargo-specific coiled-coil adaptor. However, how dynein and dynactin recognize diverse adaptors, how they interact with each other during complex formation, and the role of critical regulators such as lissencephaly-1 (LIS1) protein (LIS1) remain unclear. In this study, we determined the cryo-electron microscopy structure of dynein-dynactin on microtubules with LIS1 and the lysosomal adaptor JIP3. This structure reveals the molecular basis of interactions occurring during dynein activation. We show how JIP3 activates dynein despite its atypical architecture. Unexpectedly, LIS1 binds dynactin's p150 subunit, tethering it along the length of dynein. Our data suggest that LIS1 and p150 constrain dynein-dynactin to ensure efficient complex formation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

