Sided Stimulation of Endothelial Cells Modulates Neutrophil Trafficking in an In Vitro Sepsis Model
- PMID: 38547536
- PMCID: PMC11338706
- DOI: 10.1002/adhm.202304338
Sided Stimulation of Endothelial Cells Modulates Neutrophil Trafficking in an In Vitro Sepsis Model
Abstract
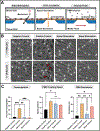
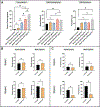
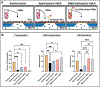
While the role of dysregulated polymorphonuclear leukocyte (PMN) transmigration in septic mediated tissue damage is well documented, strategies to mitigate aberrant transmigration across endothelium have yet to yield viable therapeutics. Recently, microphysiological systems (MPS) have emerged as novel in vitro mimetics that facilitate the development of human models of disease. With this advancement, aspects of endothelial physiology that are difficult to assess with other models can be directly probed. In this study, the role of endothelial cell (EC) apicobasal polarity on leukocyte trafficking response is evaluated with the µSiM-MVM (microphysiological system enabled by a silicon membrane - microvascular mimetic). Here, ECs are stimulated either apically or basally with a cytokine cocktail to model a septic-like challenge before introducing healthy donor PMNs into the device. Basally oriented stimulation generated a stronger PMN transmigratory response versus apical stimulation. Importantly, healthy PMNs are unable to migrate towards a bacterial peptide chemoattractant when ECs are apically stimulated, which mimics the attenuated PMN chemotaxis seen in sepsis. Escalating the apical inflammatory stimulus by a factor of five is necessary to elicit high PMN transmigration levels across endothelium. These results demonstrate that EC apicobasal polarity modulates PMN transmigratory behavior and provides insight into the mechanisms underlying sepsis.
Keywords: apicobasal polarity; membranes; microphysiological systems; neutrophil trafficking; sepsis; vascular barriers.
© 2024 Wiley‐VCH GmbH.
Conflict of interest statement
Conflict of Interest
J.L.M. cofounded SiMPore and holds an equity interest in the company. SiMPore is a company that focuses on commercializing ultrathin silicon-based technologies for a variety of purposes including the devices used in this publication.
Figures



Similar articles
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Endothelial cell apicobasal polarity coordinates distinct responses to luminally versus abluminally delivered TNF-α in a microvascular mimetic.Integr Biol (Camb). 2020 Nov 18;12(11):275-289. doi: 10.1093/intbio/zyaa022. Integr Biol (Camb). 2020. PMID: 33164044 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The fibrin-derived peptide FX06 protects human pulmonary endothelial cells against the COVID-19-triggered cytokine storm.Front Immunol. 2025 Jun 19;16:1591860. doi: 10.3389/fimmu.2025.1591860. eCollection 2025. Front Immunol. 2025. PMID: 40612940 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Fluid flow impacts endothelial-monocyte interactions in a model of vascular inflammatory fibrosis.Sci Rep. 2025 Jan 25;15(1):3227. doi: 10.1038/s41598-025-85987-z. Sci Rep. 2025. PMID: 39863621 Free PMC article.
References
-
- Nduka OO and Parrillo JE, Critical Care Clinics, 2009, 25, 677–702. - PubMed
-
- Martin GS, Mannino DM, Eaton S and Moss M, N Engl J Med, 2003, 348, 1546–1554. - PubMed
-
- Rubens M, Saxena A, Ramamoorthy V, Das S, Khera R, Hong J, Armaignac D, Veledar E, Nasir K and Gidel L, J Intensive Care Med, 2020, 35, 858–868. - PubMed
-
- Fink MP and Warren HS, Nature Reviews Drug Discovery, 2014, 13, 741–758. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

