A Validated Risk Stratification That Incorporates MAGIC Biomarkers Predicts Long-Term Outcomes in Pediatric Patients with Acute GVHD
- PMID: 38548227
- PMCID: PMC11139591
- DOI: 10.1016/j.jtct.2024.03.022
A Validated Risk Stratification That Incorporates MAGIC Biomarkers Predicts Long-Term Outcomes in Pediatric Patients with Acute GVHD
Abstract
Acute graft versus host disease (GVHD) is a common and serious complication of allogeneic hematopoietic cell transplantation (HCT) in children but overall clinical grade at onset only modestly predicts response to treatment and survival outcomes. Two tools to assess risk at initiation of treatment were recently developed. The Minnesota risk system stratifies children for risk of nonrelapse mortality (NRM) according to the pattern of GVHD target organ severity. The Mount Sinai Acute GVHD International Consortium (MAGIC) algorithm of 2 serum biomarkers (ST2 and REG3α) predicts NRM in adult patients but has not been validated in a pediatric population. We aimed to develop and validate a system that stratifies children at the onset of GVHD for risk of 6-month NRM. We determined the MAGIC algorithm probabilities (MAPs) and Minnesota risk for a multicenter cohort of 315 pediatric patients who developed GVHD requiring treatment with systemic corticosteroids. MAPs created 3 risk groups with distinct outcomes at the start of treatment and were more accurate than Minnesota risk stratification for prediction of NRM (area under the receiver operating curve (AUC), .79 versus .62, P = .001). A novel model that combined Minnesota risk and biomarker scores created from a training cohort was more accurate than either biomarkers or clinical systems in a validation cohort (AUC .87) and stratified patients into 2 groups with highly different 6-month NRM (5% versus 38%, P < .001). In summary, we validated the MAP as a prognostic biomarker in pediatric patients with GVHD, and a novel risk stratification that combines Minnesota risk and biomarker risk performed best. Biomarker-based risk stratification can be used in clinical trials to develop more tailored approaches for children who require treatment for GVHD.
Keywords: Acute GVHD; Biomarkers; Pediatric; Validation.
Copyright © 2024 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest:
MQ: Honoraria; Novartis, Vertex. ZD: Research funding: Incyte Corp., Regimmune Corp., and Taiho Oncology, Inc; Consultancy: Sanofi, Incyte Corp., MorphoSys AG, Inhibrx, PharmaBiome AG, and Ono Pharmaceutical. SAG: study support; Novartis, Kite, Cellectis, Vertex, and Servier; consult/advisory boards; Novartis, AmerisourceBergen, Eureka, Jazz, Adaptimmune, Juno, Vertex, Kyttaro, Allogene, and Cabaletta. CLK: Advisory Boards; Horizon Therapeutics, Incyte. PM: Advisory Board: SOBI, Pfizer. Consultancy: Miltenyi, Amgen, MEDAC. JEL: Consultancy fees: bluebird bio, Editas, Equillium, Inhibrx, Kamada, Mesoblast, Sanofi, and X4 Pharmaceuticals. MAP: Advisory Boards; Pfizer, Cargo, Novartis, Gentibio, Bluebird. Study support; Adaptive, Miltenyi. MW: Honoraria: Novartis, Amgen. JLMF and JEL are inventors of a GVHD biomarkers patent and receive royalties from Viracor. The remaining authors declare no competing financial interests.
Figures


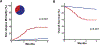
References
-
- Faraci M, Caviglia I, Biral E, et al. Acute graft-versus-host disease in pediatric allogeneic hematopoietic stem cell transplantation. Single-center experience during 10 yr. Pediatr Transplant. 2012;16:887–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

