Contribution of pudendal nerve injury to stress urinary incontinence in a male rat model
- PMID: 38548832
- PMCID: PMC10978927
- DOI: 10.1038/s41598-024-57493-1
Contribution of pudendal nerve injury to stress urinary incontinence in a male rat model
Abstract
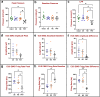
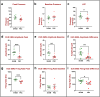
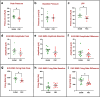
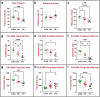
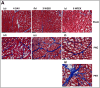
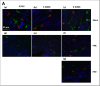
Urinary incontinence is a common complication following radical prostatectomy, as the surgery disturbs critical anatomical structures. This study explored how pudendal nerve (PN) injury affects urinary continence in male rats. In an acute study, leak point pressure (LPP) and external urethral sphincter electromyography (EMG) were performed on six male rats with an intact urethra, the urethra exposed (UE), the PN exposed (NE), and after PN transection (PNT). In a chronic study, LPP and EMG were tested in 67 rats 4 days, 3 weeks, or 6 weeks after sham PN injury, PN crush (PNC), or PNT. Urethras were assessed histologically. Acute PNT caused a significant decrease in LPP and EMG amplitude and firing rate compared to other groups. PNC resulted in a significant reduction in LPP and EMG firing rate 4 days, 3 weeks, and 6 weeks later. EMG amplitude was also significantly reduced 4 days and 6 weeks after PNC. Neuromuscular junctions were less organized and less innervated after PNC or PNT at all timepoints compared to sham injured animals. Collagen infiltration was significantly increased after PNC and PNT compared to sham at all timepoints. This rat model could facilitate preclinical testing of neuroregenerative therapies for post-prostatectomy incontinence.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Long-term effects of simulated childbirth injury on function and innervation of the urethra.Neurourol Urodyn. 2015 Apr;34(4):381-6. doi: 10.1002/nau.22561. Epub 2014 Feb 5. Neurourol Urodyn. 2015. PMID: 24501018 Free PMC article.
-
Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function.Neurourol Urodyn. 2009;28(3):229-35. doi: 10.1002/nau.20632. Neurourol Urodyn. 2009. PMID: 18973146 Free PMC article.
-
Combination histamine and serotonin treatment after simulated childbirth injury improves stress urinary incontinence.Neurourol Urodyn. 2016 Aug;35(6):703-10. doi: 10.1002/nau.22793. Epub 2015 Jun 17. Neurourol Urodyn. 2016. PMID: 26088829
-
Neurogenic aspects of stress urinary incontinence.Curr Opin Obstet Gynecol. 2010 Oct;22(5):425-9. doi: 10.1097/GCO.0b013e32833e499d. Curr Opin Obstet Gynecol. 2010. PMID: 20706117 Free PMC article. Review.
-
Pathophysiology of urinary incontinence in murine models.Int J Urol. 2013 Jan;20(1):64-71. doi: 10.1111/j.1442-2042.2012.03225.x. Epub 2012 Nov 6. Int J Urol. 2013. PMID: 23126617 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

