Taurine reduces microglia activation in the brain of aged senescence-accelerated mice by increasing the level of TREM2
- PMID: 38548872
- PMCID: PMC10978912
- DOI: 10.1038/s41598-024-57973-4
Taurine reduces microglia activation in the brain of aged senescence-accelerated mice by increasing the level of TREM2
Abstract
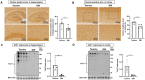
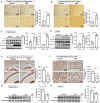
Alzheimer's disease (AD), a chronic neurodegenerative disorder, is the leading cause of dementia. Over-activated microglia is related to amyloid-beta (Aβ) and phosphorylated tau (phospho-tau) accumulation in the AD brain. Taurine is an amino acid with multiple physiological functions including anti-inflammatory effects, and has been reported to be neuroprotective in AD. However, the role of taurine in microglia-mediated AD remains unclear. Here, we examined the effects of taurine on the brains of senescence-accelerated mouse prone 8 (SAMP8) mice by comparing those administered 1% taurine water with those administered distilled water (DW). We observed increased levels of taurine and taurine transporter (TAUT) in the brains of the taurine-treated mice compared with those of control mice. Immunohistochemical and Western blot analyses revealed that taurine significantly reduced the number of activated microglia, levels of phospho-tau and Aβ deposit in the hippocampus and cortex. Triggering receptors expressed on myeloid cells-2 (TREM2) are known to protect against AD pathogenesis. Taurine upregulated TREM2 expression in the hippocampus and cortex. In conclusion, the present study suggests that taurine treatment may upregulate TREM2 to protect against microglia over-activation by decreasing the accumulation of phospho-tau and Aβ; providing an insight into a novel preventive strategy in AD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

