Primary analysis of a prospective cohort study of Japanese patients with plasma cell neoplasms in the novel drug era (2016-2021)
- PMID: 38548963
- PMCID: PMC11136844
- DOI: 10.1007/s12185-024-03754-8
Primary analysis of a prospective cohort study of Japanese patients with plasma cell neoplasms in the novel drug era (2016-2021)
Abstract
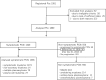
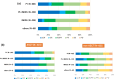
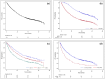
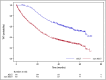
The emergence of novel drugs has significantly improved outcomes of patients with plasma cell neoplasms (PCN). The Japanese Society of Hematology conducted a prospective observational study in newly diagnosed PCN patients between 2016 and 2021. The analysis focused on 1385 patients diagnosed with symptomatic PCN between 2016 and 2018. The primary endpoint was the 3-year overall survival (OS) rate among patients requiring treatment (n = 1284), which was 70.0% (95%CI 67.4-72.6%). Approximately 94% of these patients received novel drugs as frontline therapy. The 3-year OS rate was 90.3% (95%CI 86.6-93.1%) in the 25% of patients who received upfront autologous stem cell transplantation (ASCT), versus just 61.4% (95%CI 58.0-64.6%) in those who did not receive upfront ASCT. The only unfavorable prognostic factor that affected OS in ASCT recipients was an age of 65 or higher. For patients who did not receive ASCT, independent unfavorable prognostic factors included frontline treatment with conventional chemotherapies, international staging system score of 2/3, extramedullary tumors, and Freiberg comorbidity index of 2/3. This study unequivocally demonstrates that use of novel drugs improved OS in Japanese myeloma patients, and underscores the continued importance of upfront ASCT as the standard of care in the era of novel drugs.
Keywords: Novel drug era; Plasma cell neoplasms (PCN); Prospective cohort study; Three-year overall survival rate.
© 2024. The Author(s).
Conflict of interest statement
HS reports honoraria from Takeda, Ono, Fujimoto, Janssen, Chugai, Eisai, Sanofi, AstraZeneca, Meiji Seika Pharma, and AbbVie, research funding from Ono, Bristol Myers Squibb, and AbbVie. HH reports honoraria from Janssen, Bristol Myers Squibb, and Takeda, research funding from Bristol Myers Squibb and Takeda. AY reports honoraria from Kyowa Kirin, Chugai, Janssen, Sanofi, Bristol Myers Squibb, Eisai, Takeda, and Nippon-Shinyaku, research funding from Kyowa Kirin. SK reports honoraria from Eisai, Mundipharma, Chugai, Otsuka, Sanofi, Meiji Seika Pharma, AbbVie, Ono, Janssen, Takeda, Amgen, Novartis, and Nippon-Shinyaku. SO reports honoraria from Bristol Myers Squibb, Takeda, AstraZeneca, Janssen, and Sanofi. MY reports honoraria from Takeda, Sanofi, Novartis, Bristol Myers Squibb, Meiji Seika Pharma, and Daiichi Sankyo. SF reports honoraria from Takeda, Sanofi, Janssen, Ono, and Bristol Myers Squibb. TS reports honoraria from Ono, Otsuka, Eisai, SymBio, Takeda, Chugai, Sumitomo, Daiichi Sankyo, MSD, and PharmaEssentia. KM reports honoraria from Kyowa Kirin, Chugai, MSD, Teijin Pharma, Nippon-Shinyaku, Taiho, Takeda, Otsuka, Nippon Kayaku, Daiichi Sankyo, Sumitomo, Novartis, Pfizer, Celgene, Bristol Myers Squibb, Mochida, AbbVie, Alexion, Ono, Sanofi, Janssen, SymBio, AstraZeneca, CSL Bering, Amgen, Fujimoto, Eisai, Meiji Seika Pharma, Asahi Kasei, and Astellas, research funding from Kyowa Kirin, Chugai, MSD, Teijin Pharma, Nippon-Shinyaku, Taiho, Takeda, Otsuka, Nippon Kayaku, Daiichi Sankyo, and Sumitomo, advisory board fees from Bristol Myers Squibb, Novartis, and Otsuka, travel expense from Kyowa Kirin. HT reports honoraria from Sanofi, Ono, Janssen, Takeda, Bristol Myers Squibb, and Chugai, research funding from Asahi Kasei. MA reports honoraria from Takeda, Bristol Myers Squibb, Janssen, Sanofi, Ono, and Daiichi Sankyo. HI reports honoraria from Sanofi, SymBio, Janssen, Daiichi Sankyo, Chugai, Takeda, AstraZeneca, AbbVie, and Kyowa Kirin. JK reports honoraria from Janssen, Bristol Myers Squibb, Ono, Chugai, Takeda, Sanofi, and Kyowa Kirin, research funding from Kyowa Kirin, Mochida, Sanofi, Chugai, Bristol Myers Squibb, Takeda, and Ono, consulting fees from Janssen and Bristol Myers Squibb. HT reports honoraria from Janssen, Ono, Sanofi, and Bristol Myers Squibb, consulting fee from SRL, research funding from Bristol Myers Squibb. KS reports honoraria from Ono, Bristol Myers Squibb, Janssen, and Sanofi, research funding from Ono, Celgene, AbbVie, Takeda, Sanofi, Bristol Myers Squibb, GSK, Chugai, Otsuka, Janssen, MSD, Novartis, Pfizer, and Kyowa Kirin. MK reports honoraria from Bristol Myers Squibb, Novartis, Janssen, MSD, Nippon-Shinyaku, Ono, Takeda, and Sumitomo, research funding from Ono, Takeda, Kyowa Kirin, Chugai, and Daiichi Sankyo. TI reports honoraria from Takeda, Sanofi, Janssen, Bristol Myers Squibb, and Ono, research funding from Takeda, Pfizer, Sanofi, Janssen, and Bristol Myers Squibb. IM reports honoraria from Bristol Myers Squibb, Novartis, Otsuka, Pfizer, Janssen, Astellas, Takeda, Daiichi Sankyo, Ono, Chugai, AstraZeneca, SymBio, AbbVie, and Amgen, research funding from Ono, Janssen, Nippon-Shinyaku, Kyowa Kirin, Sumitomo, Shionogi, Teijin Pharma, Boehringer Ingelheim, Sanofi, Chugai, Eisai, MSD, Asahi Kasei, Astellas, Takeda, Nihon Pharma, Daiichi Sankyo, AbbVie, Taiho, Mitsubishi Tanabe, Nippon Kayaku, CSL Behring, Mundipharma, Ayumi Pharma, Eli Lilly Japan, Actelion Pharma, and Amgen, consulting fee from Otsuka. KA reports honoraria from AbbVie, Eisai, Ono, Kyowa Kirin, Chugai, Pfizer, Bristol Myers Squibb, and Janssen, research funding from Otsuka, Kyowa Kirin, Asahi Kasei, AbbVie, Chugai, and Takeda. SI reports honoraria from Janssen, Bristol Myers Squibb, Takeda, Sanofi, Ono, and Pfizer, research funding from Chugai, Janssen, Bristol Myers Squibb, Sanofi, Daiichi Sankyo, Pfizer, Celgene, Novartis, Ono, Takeda, Amgen, Shionogi, AbbVie, Otsuka, Kyowa Kirin, and GSK, advisory board fees from Janssen, Bristol Myers Squibb, Pfizer, Sanofi, AbbVie, Otsuka, Novartis, and Takeda. All the other authors have no conflicts of interest to disclose.
Figures

References
-
- The latest statistics of the data from the Japan national cancer registry. http://ganjoho.jp/public/statistics/
-
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Esseltine DL, Liu K, Cakana A, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–2266. doi: 10.1200/JCO.2009.26.0638. - DOI - PubMed
-
- Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H, Bahlis N, Banos A, Tiab M, Delforge M, Cavenagh J, Geraldes C, Lee JJ, Chen C, Oriol A, de la Rubia J, Qiu L, White DJ, Binder D, Anderson K, Fermand JP, Moreau P, Attal M, Knight R, Chen G, Van Oostendorp J, Jacques C, Ervin-Haynes A, Avet-Loiseau H, Hulin C, Facon T, FIRST Trial Team Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi: 10.1056/NEJMoa1402551. - DOI - PubMed
-
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Chiu C, Wang J, Carson R, Crist W, Deraedt W, Nguyen H, Qi M, San-Miguel J, ALCYONE Trial Investigators Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. - DOI - PubMed
-
- Fujisaki T, Ishikawa T, Takamatsu H, Suzuki K, Min CK, Lee JH, Wang J, Carson R, Crist W, Qi M, Nagafuji K. Daratumumab plus bortezomib, melphalan, and prednisone in East Asian patients with non-transplant multiple myeloma: subanalysis of the randomized phase 3 ALCYONE trial. Ann Hematol. 2019;98(12):2805–2814. doi: 10.1007/s00277-019-03794-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

