Unidirectional gene delivery electrospun fibrous membrane via charge repulsion for tendon repair
- PMID: 38549775
- PMCID: PMC10972767
- DOI: 10.1016/j.bioactmat.2024.03.008
Unidirectional gene delivery electrospun fibrous membrane via charge repulsion for tendon repair
Abstract
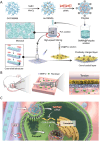
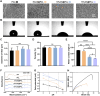
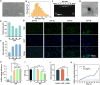
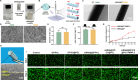
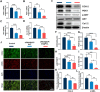
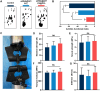
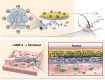
Gene therapy is capable of efficiently regulating the expression of abnormal genes in diseased tissues and expected to be a therapeutic option for refractory diseases. However, unidirectional targeting gene therapy is always desired at the tissue interface. In this study, inspired by the principle that like charges repulse each other, a positively charged micro-nano electrospun fibrous membrane with dual-layer structure was developed by electrospinning technology to achieve unidirectional delivery of siRNA-loaded cationic nanocarriers, thus realizing unidirectional gene therapy at the tendon-paratenon interface. Under the charge repulsion of positively charged layer, more cationic COX-2 siRNA nanocarriers were enriched in peritendinous tissue, which not only improved the bioavailability of the gene drug to prevent the peritendinous adhesion formation, but also avoided adverse effects on the fragile endogenous healing of tendon itself. In summary, this study provides an innovative strategy for unidirectional targeting gene therapy of tissue interface diseases by utilizing charge repulsion to facilitate unidirectional delivery of gene drugs.
Keywords: Charge repulsion; Gene therapy; Tendon repair; Unidirectional delivery.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
An Integrative Dual-Layer Poly-L-Lactic Acid Fibrous Membrane Prevents Peritendinous Adhesions.Front Bioeng Biotechnol. 2020 May 5;8:387. doi: 10.3389/fbioe.2020.00387. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32478044 Free PMC article.
-
Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing.Acta Biomater. 2017 Oct 1;61:204-216. doi: 10.1016/j.actbio.2017.07.044. Epub 2017 Aug 1. Acta Biomater. 2017. PMID: 28778532
-
Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes.Int J Nanomedicine. 2014 May 14;9:2373-85. doi: 10.2147/IJN.S59536. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24868155 Free PMC article.
-
Electrospun fibers: an innovative delivery method for the treatment of bone diseases.Expert Opin Drug Deliv. 2020 Jul;17(7):993-1005. doi: 10.1080/17425247.2020.1767583. Epub 2020 May 19. Expert Opin Drug Deliv. 2020. PMID: 32394737 Review.
-
Electrospun-Fibrous-Architecture-Mediated Non-Viral Gene Therapy Drug Delivery in Regenerative Medicine.Polymers (Basel). 2022 Jun 29;14(13):2647. doi: 10.3390/polym14132647. Polymers (Basel). 2022. PMID: 35808692 Free PMC article. Review.
Cited by
-
Early activity after strong sutures helps to tendon healing in a rat tendon rupture model.Sci Rep. 2025 Jan 2;15(1):513. doi: 10.1038/s41598-024-84393-1. Sci Rep. 2025. PMID: 39747621 Free PMC article.
-
Myeloid Cells and Sensory Nerves Mediate Peritendinous Adhesion Formation via Prostaglandin E2.Adv Sci (Weinh). 2024 Oct;11(40):e2405367. doi: 10.1002/advs.202405367. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39207041 Free PMC article.
-
Preparation Methods and Multifunctional Applications of Functionalized Electrospun Nanofibers for Biomedicine.Nanomaterials (Basel). 2025 Jun 11;15(12):909. doi: 10.3390/nano15120909. Nanomaterials (Basel). 2025. PMID: 40559272 Free PMC article. Review.
References
-
- Hurley E.A., Hull D., Shriver S.P. The next phase of human gene-therapy oversight. N. Engl. J. Med. 2019;380:401–402. - PubMed
-
- Alshaer W., Zureigat H., Al Karaki A., Al-Kadash A., Gharaibeh L., Ma'mon M.H., Aljabali A.A., Awidi A. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021;905:174–178. - PubMed
-
- Santos H.S., Rodrigues L., Vera L.N., Poletto E., Filippi-Chiela E., dos Santos Bruschi L.F.R., Schuh R., Baldo G. In situ gene therapy. Curr. Gene Ther. 2021;21:406–430. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

