Chiral Isochalcogenourea-Catalysed Enantioselective (4+2) Cycloadditions of Allenoates
- PMID: 38549953
- PMCID: PMC10976662
- DOI: 10.1002/ange.202315345
Chiral Isochalcogenourea-Catalysed Enantioselective (4+2) Cycloadditions of Allenoates
Abstract
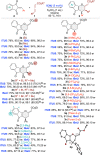
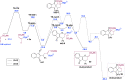
Allenoates are versatile building blocks which are primarily activated and controlled using chiral tert. phosphine and tert. amine Lewis bases. We herein report the first example of allenoate activation by using chiral isochalcogenoureas (IChU) for formal (4+2) cycloaddition reactions. Compared to established phosphine and amine catalysis, the use of these easily available Lewis bases enables new stereoselective reaction pathways proceeding with high enantioselectivities, diastereoselectivities, and in good yields. In addition, the factors governing enantioselectivity and the origin of the observed differences compared to other commonly used Lewis bases are explained.
Chiral isochalcogenoureas (IChU) were successfully established as powerful organocatalysts for the activation and control of allenoates. This unprecedented strategy allows for unique reactivities and high levels of stereocontrol, facilitating reaction pathways that are not possible with the existing methods.
Keywords: Allenoates; Cycloadditions; DFT Calculations; Lewis Bases; Organocatalysis.
© 2023 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
