Continuous flow synthesis enabling reaction discovery
- PMID: 38550700
- PMCID: PMC10967013
- DOI: 10.1039/d3sc06808k
Continuous flow synthesis enabling reaction discovery
Abstract
This article defines the role that continuous flow chemistry can have in new reaction discovery, thereby creating molecular assembly opportunities beyond our current capabilities. Most notably the focus is based upon photochemical, electrochemical and temperature sensitive processes where continuous flow methods and machine assisted processing can have significant impact on chemical reactivity patterns. These flow chemical platforms are ideally placed to exploit future innovation in data acquisition, feed-back and control through artificial intelligence (AI) and machine learning (ML) techniques.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




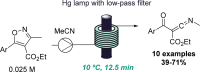
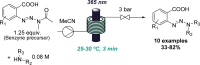
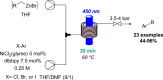




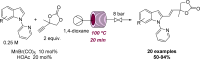


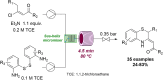




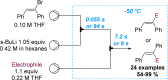
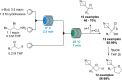
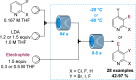


References
-
- Herges R. Tetrahedron Comput. Methodol. 1988;1:15–25. doi: 10.1016/0898-5529(88)90005-X. - DOI
- Lavigne C. Gomes G. Pollice R. Aspuru-Guzik A. Chem. Sci. 2022;13:13857–13871. doi: 10.1039/D2SC05135D. - DOI - PMC - PubMed
- Bort W. Baskin I. I. Gimadiev T. Mukanov A. Nugmanov R. Sidorov P. Marcou G. Horvath D. Klimchuk O. Madzhidov T. Varnek A. Sci. Rep. 2021;11:3178. doi: 10.1038/s41598-021-81889-y. - DOI - PMC - PubMed
-
- Beach E. S. Cui Z. Anastas P. T. Energy Environ. Sci. 2009;2:1038–1049. doi: 10.1039/B904997P. - DOI
- Li C.-J. Trost B. M. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13197–13202. doi: 10.1073/pnas.0804348105. - DOI - PMC - PubMed
- Isidro-Llobet A. Kenworthy M. N. Mukherjee S. Kopach M. E. Wegner K. Gallou F. Smith A. G. Roschangar F. J. Org. Chem. 2019;84:4615–4628. doi: 10.1021/acs.joc.8b03001. - DOI - PubMed
- Kar S. Sanderson H. Roy K. Benfenati E. Leszczynski J. Chem. Rev. 2022;122(3):3637–3710. doi: 10.1021/acs.chemrev.1c00631. - DOI - PubMed
-
- Shaw M. H. Twilton J. MacMillan D. W. C. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. - DOI - PMC - PubMed
- Yoon T. P. Ischay M. A. Du J. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. - DOI - PubMed
- Kärkäs M. D. Porco Jr J. A. Stephenson C. R. J. Chem. Rev. 2016;116:9683–9747. doi: 10.1021/acs.chemrev.5b00760. - DOI - PMC - PubMed
-
- Yan M. Kawamata Y. Baran P. S. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. - DOI - PMC - PubMed
- Kingston C. Palkowitz M. D. Takahira Y. Vantourout J. C. Peters B. K. Kawamata Y. Baran P. S. Acc. Chem. Res. 2020;53:72–83. doi: 10.1021/acs.accounts.9b00539. - DOI - PMC - PubMed
- Schotten C. Nicholls T. P. Bourne R. A. Kapur N. Nguyen B. N. Willans C. E. Green Chem. 2020;22:3358–3375. doi: 10.1039/D0GC01247E. - DOI
-
- Bell E. L. Finnigan W. France S. P. Green A. P. Hayes M. A. Hepworth L. J. Lovelock S. L. Niikura H. Osuna S. Romero E. Ryan K. S. Turner N. J. Flitsch S. L. Nat. Rev. Methods Primers. 2021;1:46. doi: 10.1038/s43586-021-00044-z. - DOI
Publication types
LinkOut - more resources
Full Text Sources

