Immunogenicity and protective efficacy of inactivated coxsackievirus B4 viral particles
- PMID: 38551145
- PMCID: PMC11000607
- DOI: 10.1080/22221751.2024.2337665
Immunogenicity and protective efficacy of inactivated coxsackievirus B4 viral particles
Abstract
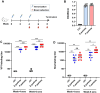
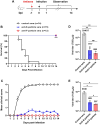
Coxsackievirus B4 (CVB4) is associated with a range of acute and chronic diseases such as hand, foot, and mouth disease, myocarditis, meningitis, pancreatitis, and type 1 diabetes, affecting millions of young children annually around the world. However, no vaccine is currently available for preventing CVB4 infection. Here, we report the development of inactivated viral particle vaccines for CVB4. Two types of inactivated CVB4 particles were prepared from CVB4-infected cell cultures as vaccine antigens, including F-particle (also called mature virion) consisting of VP1, VP3, VP2, and VP4 subunit proteins, and E-particle (also called empty capsid) which is made of VP1, VP3, and uncleaved VP0. Both the inactivated CVB4 F-particle and E-particle were able to potently elicit neutralizing antibodies in mice, despite slightly lower neutralizing antibody titres seen with the E-particle vaccine after the third immunization. Importantly, we demonstrated that passive transfer of either anti-F-particle or anti-E-particle sera could completely protect the recipient mice from lethal CVB4 challenge. Our study not only defines the immunogenicity and protective efficacy of inactivated CVB4 F-particle and E-particle but also reveals the central role of neutralizing antibodies in anti-CVB4 protective immunity, thus providing important information that may accelerate the development of inactivated CVB4 vaccines.
Keywords: Coxsackievirus B4; E-particle; F-particle; inactivated vaccine; neutralizing antibody; virus challenge.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Viral Protein VP1 Virus-like Particles (VLP) of CVB4 Induces Protective Immunity against Lethal Challenges with Diabetogenic E2 and Wild Type JBV Strains in Mice Model.Viruses. 2023 Mar 29;15(4):878. doi: 10.3390/v15040878. Viruses. 2023. PMID: 37112858 Free PMC article.
-
Active immunization with a Coxsackievirus A16 experimental inactivated vaccine induces neutralizing antibodies and protects mice against lethal infection.Vaccine. 2013 Apr 26;31(18):2215-21. doi: 10.1016/j.vaccine.2013.03.007. Epub 2013 Mar 13. Vaccine. 2013. PMID: 23499596
-
Inactivated coxsackievirus A10 experimental vaccines protect mice against lethal viral challenge.Vaccine. 2016 Sep 22;34(41):5005-5012. doi: 10.1016/j.vaccine.2016.08.033. Epub 2016 Aug 22. Vaccine. 2016. PMID: 27562093
-
Production of EV71 vaccine candidates.Hum Vaccin Immunother. 2012 Dec 1;8(12):1775-83. doi: 10.4161/hv.21739. Epub 2012 Sep 19. Hum Vaccin Immunother. 2012. PMID: 22992566 Free PMC article. Review.
-
Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine.Adv Vet Med. 1999;41:447-61. doi: 10.1016/s0065-3519(99)80034-2. Adv Vet Med. 1999. PMID: 9890035 Review.
References
-
- Mulders MN, Salminen M, Kalkkinen N, et al. . Molecular epidemiology of coxsackievirus B4 and disclosure of the correct VP1/2Apro cleavage site: evidence for high genomic diversity and long-term endemicity of distinct genotypes. J Gen Virol. 2000 Mar;81(Pt 3):803–812. doi:10.1099/0022-1317-81-3-803 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
