An NADH/NAD+-favored aldo-keto reductase facilitates avilamycin A biosynthesis by primarily catalyzing oxidation of avilamycin C
- PMID: 38551341
- PMCID: PMC11022570
- DOI: 10.1128/aem.00150-24
An NADH/NAD+-favored aldo-keto reductase facilitates avilamycin A biosynthesis by primarily catalyzing oxidation of avilamycin C
Abstract
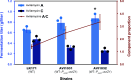
Avilamycins, which possess potent inhibitory activity against Gram-positive bacteria, are a group of oligosaccharide antibiotics produced by Streptomyces viridochromogenes. Among these structurally related oligosaccharide antibiotics, avilamycin A serves as the main bioactive component in veterinary drugs and animal feed additives, which differs from avilamycin C only in the redox state of the two-carbon branched-chain of the terminal octose moiety. However, the mechanisms underlying assembly and modification of the oligosaccharide chain to diversify individual avilamycins remain poorly understood. Here, we report that AviZ1, an aldo-keto reductase in the avilamycin pathway, can catalyze the redox conversion between avilamycins A and C. Remarkably, the ratio of these two components produced by AviZ1 depends on the utilization of specific redox cofactors, namely NADH/NAD+ or NADPH/NADP+. These findings are inspired by gene disruption and complementation experiments and are further supported by in vitro enzymatic activity assays, kinetic analyses, and cofactor affinity studies on AviZ1-catalyzed redox reactions. Additionally, the results from sequence analysis, structure prediction, and site-directed mutagenesis of AviZ1 validate it as an NADH/NAD+-favored aldo-keto reductase that primarily oxidizes avilamycin C to form avilamycin A by utilizing abundant NAD+ in vivo. Building upon the biological function and catalytic activity of AviZ1, overexpressing AviZ1 in S. viridochromogenes is thus effective to improve the yield and proportion of avilamycin A in the fermentation profile of avilamycins. This study represents, to our knowledge, the first characterization of biochemical reactions involved in avilamycin biosynthesis and contributes to the construction of high-performance strains with industrial value.IMPORTANCEAvilamycins are a group of oligosaccharide antibiotics produced by Streptomyces viridochromogenes, which can be used as veterinary drugs and animal feed additives. Avilamycin A is the most bioactive component, differing from avilamycin C only in the redox state of the two-carbon branched-chain of the terminal octose moiety. Currently, the biosynthetic pathway of avilamycins is not clear. Here, we report that AviZ1, an aldo-keto reductase in the avilamycin pathway, can catalyze the redox conversion between avilamycins A and C. More importantly, AviZ1 exhibits a unique NADH/NAD+ preference, allowing it to efficiently catalyze the oxidation of avilamycin C to form avilamycin A using abundant NAD+ in cells. Thus, overexpressing AviZ1 in S. viridochromogenes is effective to improve the yield and proportion of avilamycin A in the fermentation profile of avilamycins. This study serves as an enzymological guide for rational strain design, and the resulting high-performance strains have significant industrial value.
Keywords: aldo-keto reductase; avilamycins; biosynthesis; branched-chain sugar; rational strain design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Avilamycin production enhancement by mutagenesis and fermentation optimization in Streptomyces viridochromogenes.World J Microbiol Biotechnol. 2022 Jan 31;38(3):50. doi: 10.1007/s11274-021-03191-3. World J Microbiol Biotechnol. 2022. PMID: 35098381
-
Transient-state and steady-state kinetic studies of the mechanism of NADH-dependent aldehyde reduction catalyzed by xylose reductase from the yeast Candida tenuis.Biochemistry. 2001 Aug 28;40(34):10371-81. doi: 10.1021/bi010148a. Biochemistry. 2001. PMID: 11513616
-
Biosynthesis of the orthosomycin antibiotic avilamycin A: deductions from the molecular analysis of the avi biosynthetic gene cluster of Streptomyces viridochromogenes Tü57 and production of new antibiotics.Chem Biol. 2001 Jun;8(6):569-81. doi: 10.1016/s1074-5521(01)00040-0. Chem Biol. 2001. PMID: 11410376
-
The aldo-keto reductases (AKRs): Overview.Chem Biol Interact. 2015 Jun 5;234:236-46. doi: 10.1016/j.cbi.2014.09.024. Epub 2014 Oct 7. Chem Biol Interact. 2015. PMID: 25304492 Free PMC article. Review.
-
Comparative anatomy of the aldo-keto reductase superfamily.Biochem J. 1997 Sep 15;326 ( Pt 3)(Pt 3):625-36. doi: 10.1042/bj3260625. Biochem J. 1997. PMID: 9307009 Free PMC article. Review.
References
-
- Wright DE. 1979. Orthosomycins - new family of antibiotics. Tetrahedron 35:1207–1237. doi:10.1016/0040-4020(79)80046-0 - DOI
-
- Krupkin M, Wekselman I, Matzov D, Eyal Z, Diskin Posner Y, Rozenberg H, Zimmerman E, Bashan A, Yonath A. 2016. Avilamycin and evernimicin induce structural changes in rProteins uL16 and CTC that enhance the inhibition of A-site tRNA binding. Proc Natl Acad Sci USA 113:E6796–E6805. doi:10.1073/pnas.1614297113 - DOI - PMC - PubMed
-
- Kellerschierlein W, Heilman W, Ollis WD, Smith C. 1979. Metabolic products from microorganisms .178. avilamycin-A and avilamycin-C - chemical decomposition and spectroscopic studies. Helv Chim Acta 62:7–20. doi:10.1002/chin.197919377 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

