Retracted and republished from: "The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs"
- PMID: 38551343
- PMCID: PMC11077966
- DOI: 10.1128/mbio.00175-24
Retracted and republished from: "The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs"
Abstract
Influenza viruses (IVs) threaten global human health due to the high morbidity, infection, and mortality rates. Currently, the influenza drugs recommended by the Food and Drug Administration are oseltamivir, zanamivir, peramivir, and baloxavir marboxil. These recommended antivirals are currently effective for major subtypes of IVs as the compounds target conserved domains in neuraminidase or polymerase acidic (PA) protein. However, this trend may gradually change due to the selection of antiviral drugs and the natural evolution of IVs. Therefore, there is an urgent need to develop drugs related to the treatment of influenza to deal with the next pandemic. Here, we summarized the cutting-edge research in mechanism of action, inhibitory activity, and clinical efficacy of drugs that have been approved and drugs that are still in clinical trials for influenza treatment. We hope this review will provide up-to-date and comprehensive information on influenza antivirals and generate hypotheses for screens and development of new broad-spectrum influenza drugs in the near future.
Keywords: adverse event; antiviral drugs; clinical drugs; influenza virus; mechanism of action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
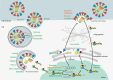

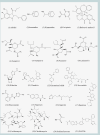

Corrected and republished from
-
The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs.mBio. 2023 Oct 31;14(5):e0127323. doi: 10.1128/mbio.01273-23. Epub 2023 Aug 23. mBio. 2023. Retraction in: mBio. 2024 Apr 10;15(4):e0010624. doi: 10.1128/mbio.00106-24. Corrected and republished in: mBio. 2024 May 8;15(5):e0017524. doi: 10.1128/mbio.00175-24. PMID: 37610204 Free PMC article. Retracted. Corrected and republished. Review.
Similar articles
-
The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs.mBio. 2023 Oct 31;14(5):e0127323. doi: 10.1128/mbio.01273-23. Epub 2023 Aug 23. mBio. 2023. Retraction in: mBio. 2024 Apr 10;15(4):e0010624. doi: 10.1128/mbio.00106-24. Corrected and republished in: mBio. 2024 May 8;15(5):e0017524. doi: 10.1128/mbio.00175-24. PMID: 37610204 Free PMC article. Retracted. Corrected and republished. Review.
-
Pharmacologic background and clinical issue of anti-influenza drugs.Fukushima J Med Sci. 2025 Jan 18;71(1):1-12. doi: 10.5387/fms.24-00029. Epub 2024 Dec 18. Fukushima J Med Sci. 2025. PMID: 39694499 Free PMC article. Review.
-
Antiviral influenza treatments and hemorrhage-related adverse events in the United States Food and Drug Administration Adverse Event Reporting System (FAERS) database.Pharmacotherapy. 2024 May;44(5):383-393. doi: 10.1002/phar.2920. Epub 2024 Apr 24. Pharmacotherapy. 2024. PMID: 38656741
-
Potential Role of Endonuclease Inhibition and Other Targets in the Treatment of Influenza.Curr Drug Targets. 2020;21(2):202-211. doi: 10.2174/1389450120666190801115130. Curr Drug Targets. 2020. PMID: 31368872 Review.
-
Effect of Baloxavir and Oseltamivir in Combination on Infection with Influenza Viruses with PA/I38T or PA/E23K Substitutions in the Ferret Model.mBio. 2022 Aug 30;13(4):e0105622. doi: 10.1128/mbio.01056-22. Epub 2022 Aug 8. mBio. 2022. PMID: 35938724 Free PMC article.
Cited by
-
A Cyclic Peptide Based on Pheasant Cathelicidin Inhibits Influenza A H1N1 Virus Infection.Antibiotics (Basel). 2024 Jun 28;13(7):606. doi: 10.3390/antibiotics13070606. Antibiotics (Basel). 2024. PMID: 39061288 Free PMC article.
-
Inhibition of RAN attenuates influenza a virus replication and nucleoprotein nuclear export.Emerg Microbes Infect. 2024 Dec;13(1):2387910. doi: 10.1080/22221751.2024.2387910. Epub 2024 Aug 12. Emerg Microbes Infect. 2024. PMID: 39087696 Free PMC article.
-
Influenza, SARS-CoV-2, and Their Impact on Chronic Lung Diseases and Fibrosis: Exploring Therapeutic Options.Am J Pathol. 2024 Oct;194(10):1807-1822. doi: 10.1016/j.ajpath.2024.06.004. Epub 2024 Jul 18. Am J Pathol. 2024. PMID: 39032604 Review.
-
Catch, Cut, or Block? Versatile 4-N-Derivatized Sialyl Glycosides for Influenza Virus Neuraminidase Detection and Purification.Angew Chem Int Ed Engl. 2025 Jun 17;64(25):e202505903. doi: 10.1002/anie.202505903. Epub 2025 Apr 21. Angew Chem Int Ed Engl. 2025. PMID: 40210607 Free PMC article.
References
-
- WHO . 2023. Influenza (seasonal). Available from: https://www.who.int/health-topics/influenza-seasonal
-
- CDC . 2023. Specific avian flu viruses. Available from: https://www.cdc.gov/flu/avianflu/specific-flu-viruses.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

