Lessons learnt from the multi-centre LAparoscopic Versus Abdominal hysterectomy (LAVA) randomised controlled trial
- PMID: 38551473
- PMCID: PMC11198880
- DOI: 10.52054/FVVO.16.1.003
Lessons learnt from the multi-centre LAparoscopic Versus Abdominal hysterectomy (LAVA) randomised controlled trial
Abstract
Background: The LAparoscopic Versus Abdominal hysterectomy (LAVA) randomised controlled trial comparing laparoscopic hysterectomy (LH) and abdominal hysterectomy (AH) closed prematurely on the grounds of futility. Here we identify the challenges faced and lessons learnt.
Objectives: To explore the views and experiences of clinical/research staff in order to understand how these might act as barriers to trial participation and recruitment.
Materials and methods: Review of the trial progress and collation of the views and experiences of clinical/ research staff on all aspects of the trial. Data were collected from transcribed conversations, email, phone, or video conferencing interactions and analysed descriptively.
Main outcome measures: Site set-up milestones, recruitment rates and reasons provided by clinical/research staff for site's declining to participate. Opinions, preferences and experiences of clinicians/researchers and challenges to participation and recruitment.
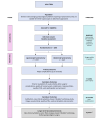
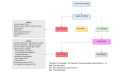
Results: The mean time from initial site contact to opening was 253 days and 68 days to randomise their first participant. 265 patients were screened from 13 sites over 13 months, 154 were eligible, and 75 (59%) were randomised. Of the 53 not randomised, 23 (43%) women preferred LH whilst 6 (11%) preferred AH. The main reasons given for failure to recruit or activate set-up in the 21 sites open or in set-up, were lack of research/ clinical capacity imposed by the COVID-19 pandemic and lack of clinician equipoise.
Conclusions: The main reasons for the LAVA trial failure were lack of equipoise amongst surgeons and the adverse impact of the COVID-19 pandemic on clinical/research services.
What is new?: Surgeons' preference for laparoscopic hysterectomy is not shared by most patients. Many patients prefer an open hysterectomy to a laparoscopic one.
Figures
Similar articles
-
Liver resection surgery compared with thermal ablation in high surgical risk patients with colorectal liver metastases: the LAVA international RCT.Health Technol Assess. 2020 Apr;24(21):1-38. doi: 10.3310/hta24210. Health Technol Assess. 2020. PMID: 32370822 Free PMC article. Clinical Trial.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.Health Technol Assess. 2004 Jun;8(26):1-154. doi: 10.3310/hta8260. Health Technol Assess. 2004. PMID: 15215018 Clinical Trial.
-
Surgery versus conservative management of stable thoracolumbar fracture: the PRESTO feasibility RCT.Health Technol Assess. 2021 Nov;25(62):1-126. doi: 10.3310/hta25620. Health Technol Assess. 2021. PMID: 34780323 Clinical Trial.
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
-
Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD000329. doi: 10.1002/14651858.CD000329.pub4. Cochrane Database Syst Rev. 2021. PMID: 33619722 Free PMC article.
Cited by
-
Feasibility, acceptability and appropriateness of laparoscopic versus abdominal hysterectomy for women and healthcare professionals: the LAVA trial qualitative process evaluation.Health Technol Assess. 2025 Jul 23:1-21. doi: 10.3310/GJTC1325. Online ahead of print. Health Technol Assess. 2025 Jul 23:1-21. doi: 10.3310/GJTC1325. Online ahead of print. PMID: 40717555
-
The glass ceiling of endometriosis surgeons is research.Facts Views Vis Obgyn. 2024 Mar;16(1):1-3. doi: 10.52054/FVVO.16.1.011. Facts Views Vis Obgyn. 2024. PMID: 38551469 Free PMC article. No abstract available.
-
Comparison of complications and recovery after laparoscopic and abdominal hysterectomy for benign disease: the LAparoscopic Versus Abdominal hysterectomy (LAVA) randomised controlled trial.BMJ Open. 2025 Jun 5;15(6):e096265. doi: 10.1136/bmjopen-2024-096265. BMJ Open. 2025. PMID: 40473285 Free PMC article. Clinical Trial.
References
-
- Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery. 2006;139:469–483. - PubMed
-
- ACOG. Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156–1158. - PubMed
-
- Campbell MK, Snowdon C, Francis D, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11:iii,ix–105. - PubMed
LinkOut - more resources
Full Text Sources