LET-767 determines lipid droplet protein targeting and lipid homeostasis
- PMID: 38551495
- PMCID: PMC10982117
- DOI: 10.1083/jcb.202311024
LET-767 determines lipid droplet protein targeting and lipid homeostasis
Abstract
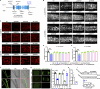
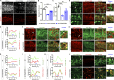
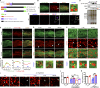
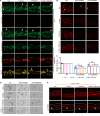
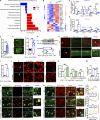
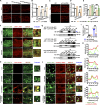
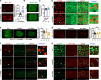
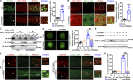
Lipid droplets (LDs) are composed of a core of neutral lipids wrapped by a phospholipid (PL) monolayer containing several hundred proteins that vary between different cells or organisms. How LD proteins target to LDs is still largely unknown. Here, we show that RNAi knockdown or gene mutation of let-767, encoding a member of hydroxysteroid dehydrogenase (HSD), displaced the LD localization of three well-known LD proteins: DHS-3 (dehydrogenase/reductase), PLIN-1 (perilipin), and DGAT-2 (diacylglycerol O-acyltransferase 2), and also prevented LD growth in Caenorhabditis elegans. LET-767 interacts with ARF-1 (ADP-ribosylation factor 1) to prevent ARF-1 LD translocation for appropriate LD protein targeting and lipid homeostasis. Deficiency of LET-767 leads to the release of ARF-1, which further recruits and promotes translocation of ATGL-1 (adipose triglyceride lipase) to LDs for lipolysis. The displacement of LD proteins caused by LET-767 deficiency could be reversed by inhibition of either ARF-1 or ATGL-1. Our work uncovers a unique LET-767 for determining LD protein targeting and maintaining lipid homeostasis.
© 2024 Fu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures





Similar articles
-
Hydroxysteroid dehydrogenase family proteins on lipid droplets through bacteria, C. elegans, and mammals.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Aug;1863(8):881-894. doi: 10.1016/j.bbalip.2018.04.018. Epub 2018 Apr 25. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29702244
-
Hormone-sensitive lipase preferentially redistributes to lipid droplets associated with perilipin-5 in human skeletal muscle during moderate-intensity exercise.J Physiol. 2018 Jun;596(11):2077-2090. doi: 10.1113/JP275502. Epub 2018 Apr 11. J Physiol. 2018. PMID: 29527681 Free PMC article.
-
Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans.Mol Cell Biol. 2014 Nov 15;34(22):4165-76. doi: 10.1128/MCB.00722-14. Epub 2014 Sep 8. Mol Cell Biol. 2014. PMID: 25202121 Free PMC article.
-
Regulation of lipid droplet dynamics and lipid homeostasis by hydroxysteroid dehydrogenase proteins.Trends Cell Biol. 2025 Feb;35(2):153-165. doi: 10.1016/j.tcb.2024.10.010. Epub 2024 Nov 26. Trends Cell Biol. 2025. PMID: 39603915 Review.
-
Adding fuel to the fire: The lipid droplet and its associated proteins in cancer progression.Int J Biol Sci. 2022 Oct 17;18(16):6020-6034. doi: 10.7150/ijbs.74902. eCollection 2022. Int J Biol Sci. 2022. PMID: 36439875 Free PMC article. Review.
Cited by
-
AMPK regulates ARF1 localization to membrane contact sites to facilitate fatty acid transfer between lipid droplets and mitochondria.Cell Death Dis. 2025 Aug 18;16(1):623. doi: 10.1038/s41419-025-07957-7. Cell Death Dis. 2025. PMID: 40825999 Free PMC article.
References
-
- Blanchette-Mackie, E.J., Dwyer N.K., Barber T., Coxey R.A., Takeda T., Rondinone C.M., Theodorakis J.L., Greenberg A.S., and Londos C.. 1995. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 36:1211–1226. 10.1016/S0022-2275(20)41129-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

