Subpopulation commensalism promotes Rac1-dependent invasion of single cells via laminin-332
- PMID: 38551497
- PMCID: PMC10982113
- DOI: 10.1083/jcb.202308080
Subpopulation commensalism promotes Rac1-dependent invasion of single cells via laminin-332
Abstract
Phenotypic heterogeneity poses a significant hurdle for cancer treatment but is under-characterized in the context of tumor invasion. Amidst the range of phenotypic heterogeneity across solid tumor types, collectively invading cells and single cells have been extensively characterized as independent modes of invasion, but their intercellular interactions have rarely been explored. Here, we isolated collectively invading cells and single cells from the heterogeneous 4T1 cell line and observed extensive transcriptional and epigenetic diversity across these subpopulations. By integrating these datasets, we identified laminin-332 as a protein complex exclusively secreted by collectively invading cells. Live-cell imaging revealed that laminin-332 derived from collectively invading cells increased the velocity and directionality of single cells. Despite collectively invading and single cells having similar expression of the integrin α6β4 dimer, single cells demonstrated higher Rac1 activation upon laminin-332 binding to integrin α6β4. This mechanism suggests a novel commensal relationship between collectively invading and single cells, wherein collectively invading cells promote the invasive potential of single cells through a laminin-332/Rac1 axis.
© 2024 Yoon et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

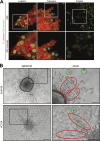

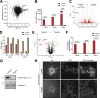
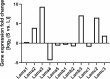




Comment in
-
A collective strategy to promote the dissemination of single cancer cells.J Cell Biol. 2024 Jun 3;223(6):e202405014. doi: 10.1083/jcb.202405014. Epub 2024 May 8. J Cell Biol. 2024. PMID: 38717454 Free PMC article.
Similar articles
-
The metastasis-associated molecule C4.4A promotes tissue invasion and anchorage independence by associating with the alpha6beta4 integrin.Mol Oncol. 2013 Oct;7(5):917-28. doi: 10.1016/j.molonc.2013.05.002. Epub 2013 May 15. Mol Oncol. 2013. PMID: 23727360 Free PMC article.
-
Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization.J Biol Chem. 2006 Nov 17;281(46):35487-98. doi: 10.1074/jbc.M606317200. Epub 2006 Sep 14. J Biol Chem. 2006. PMID: 16973601 Free PMC article.
-
α1β1 integrin/Rac1-dependent mesangial invasion of glomerular capillaries in Alport syndrome.Am J Pathol. 2013 Oct;183(4):1269-1280. doi: 10.1016/j.ajpath.2013.06.015. Epub 2013 Aug 2. Am J Pathol. 2013. PMID: 23911822 Free PMC article.
-
The opposing roles of laminin-binding integrins in cancer.Matrix Biol. 2017 Jan;57-58:213-243. doi: 10.1016/j.matbio.2016.08.007. Epub 2016 Aug 22. Matrix Biol. 2017. PMID: 27562932 Review.
-
Role of α6β4 integrin in cell motility, invasion and metastasis of mammary tumors.Curr Protein Pept Sci. 2011 Feb;12(1):23-9. doi: 10.2174/138920311795659399. Curr Protein Pept Sci. 2011. PMID: 21190525 Review.
Cited by
-
Collective migration modes in development, tissue repair and cancer.Nat Rev Mol Cell Biol. 2025 Jun 5. doi: 10.1038/s41580-025-00858-9. Online ahead of print. Nat Rev Mol Cell Biol. 2025. PMID: 40473829 Review.
-
TGF-β signaling redirects Sox11 gene regulatory activity to promote partial EMT and collective invasion of oncogenically transformed intestinal organoids.Oncogenesis. 2025 May 20;14(1):17. doi: 10.1038/s41389-025-00560-7. Oncogenesis. 2025. PMID: 40393982 Free PMC article.
References
-
- Aryee, M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., and Irizarry R.A.. 2014. Minfi: A flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 30:1363–1369. 10.1093/bioinformatics/btu049 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

