Integrated analyses reveal unexpected complex inversion and recombination in RH genes
- PMID: 38551808
- PMCID: PMC11222952
- DOI: 10.1182/bloodadvances.2023012147
Integrated analyses reveal unexpected complex inversion and recombination in RH genes
Abstract
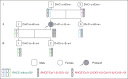
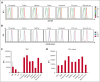
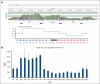
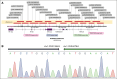
Phenotype D-- is associated with severe hemolytic transfusion reactions and hemolytic disease of the fetus and newborn. It is typically caused by defective RHCE genes. In this study, we identified a D-- phenotype proband and verified Rh phenotypes of other 6 family members. However, inconsistent results between the phenotypic analysis and Sanger sequencing revealed intact RHCE exons with no mutations in the D-- proband, but the protein was not expressed. Subsequent whole-genome sequencing by Oxford Nanopore Technologies of the proband revealed an inversion with ambiguous breakpoints in intron 2 and intron 7 and copy number variation loss in the RHCE gene region. Given that the RHCE gene is highly homologous to the RHD gene, we conducted a comprehensive analysis using Pacific Biosciences long-read target sequencing, Bionano optical genome mapping, and targeted next-generation sequencing. Our findings revealed that the proband had 2 novel recombinant RHCE haplotypes, RHCE∗Ce(1-2)-D(3-10) and RHCE∗Ce(1-2)-D(3-10)-Ce(10-8)-Ce(3-10), with clear-cut breakpoints identified. Furthermore, the RH haplotypes of the family members were identified and verified. In summary, we made, to our knowledge, a novel discovery of hereditary large inversion and recombination events occurring between the RHD and RHCE genes, leading to a lack of RhCE expression. This highlights the advantages of using integrated genetic analyses and also provides new insights into RH genotyping.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Similar articles
-
RH diversity in Mali: characterization of a new haplotype RHD*DIVa/RHCE*ceTI(D2).Transfusion. 2015 Jun;55(6 Pt 2):1423-31. doi: 10.1111/trf.13109. Epub 2015 Apr 10. Transfusion. 2015. PMID: 25857637
-
Accurate long-read sequencing allows assembly of the duplicated RHD and RHCE genes harboring variants relevant to blood transfusion.Am J Hum Genet. 2022 Jan 6;109(1):180-191. doi: 10.1016/j.ajhg.2021.12.003. Epub 2021 Dec 29. Am J Hum Genet. 2022. PMID: 34968422 Free PMC article.
-
A Tutsi family harbouring two new RHCE variant alleles and a new haplotype in the Rh blood group system.Vox Sang. 2020 Jul;115(5):451-455. doi: 10.1111/vox.12905. Epub 2020 Mar 20. Vox Sang. 2020. PMID: 32196693
-
Evidence for a separate genetic origin of the partial D phenotype DBT in a Japanese family.Transfusion. 1999 Nov-Dec;39(11-12):1259-65. doi: 10.1046/j.1537-2995.1999.39111259.x. Transfusion. 1999. PMID: 10604255
-
Five-Years Review of RHCE Alleles Detected after Weak and/or Discrepant C Results in Southern France.Genes (Basel). 2022 Jun 14;13(6):1058. doi: 10.3390/genes13061058. Genes (Basel). 2022. PMID: 35741820 Free PMC article. Review.
References
-
- ISBT: Red Cell Immunogenetics and Blood Group Terminology | ISBT Working Party | The International Society of Blood Transfusion (ISBT) https://www.isbtweb.org
-
- Matassi G, Cherif-Zahar B, Pesole G, Raynal V, Cartron JP. The members of the RH gene family (RH50 and RH30) followed different evolutionary pathways. J Mol Evol. 1999;48(2):151–159. - PubMed
-
- Zhao FY, Li Q, Zhang DM, et al. A novel silent RHCE allele in Chinese population. Transfus Med. 2019;29(6):430–433. - PubMed

