A red-emitting carborhodamine for monitoring and measuring membrane potential
- PMID: 38551837
- PMCID: PMC10998576
- DOI: 10.1073/pnas.2315264121
A red-emitting carborhodamine for monitoring and measuring membrane potential
Abstract
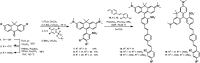
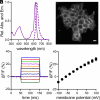
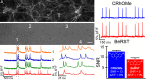
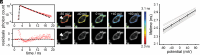
Biological membrane potentials, or voltages, are a central facet of cellular life. Optical methods to visualize cellular membrane voltages with fluorescent indicators are an attractive complement to traditional electrode-based approaches, since imaging methods can be high throughput, less invasive, and provide more spatial resolution than electrodes. Recently developed fluorescent indicators for voltage largely report changes in membrane voltage by monitoring voltage-dependent fluctuations in fluorescence intensity. However, it would be useful to be able to not only monitor changes but also measure values of membrane potentials. This study discloses a fluorescent indicator which can address both. We describe the synthesis of a sulfonated tetramethyl carborhodamine fluorophore. When this carborhodamine is conjugated with an electron-rich, methoxy (-OMe) containing phenylenevinylene molecular wire, the resulting molecule, CRhOMe, is a voltage-sensitive fluorophore with red/far-red fluorescence. Using CRhOMe, changes in cellular membrane potential can be read out using fluorescence intensity or lifetime. In fluorescence intensity mode, CRhOMe tracks fast-spiking neuronal action potentials (APs) with greater signal-to-noise than state-of-the-art BeRST 1 (another voltage-sensitive fluorophore). CRhOMe can also measure values of membrane potential. The fluorescence lifetime of CRhOMe follows a single exponential decay, substantially improving the quantification of membrane potential values using fluorescence lifetime imaging microscopy (FLIM). The combination of red-shifted excitation and emission, mono-exponential decay, and high voltage sensitivity enable fast FLIM recording of APs in cardiomyocytes. The ability to both monitor and measure membrane potentials with red light using CRhOMe makes it an important approach for studying biological voltages.
Keywords: fluorescence; imaging; physiology; voltage.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
A red-emitting carborhodamine for monitoring and measuring membrane potential.bioRxiv [Preprint]. 2023 Oct 10:2023.10.06.561080. doi: 10.1101/2023.10.06.561080. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2315264121. doi: 10.1073/pnas.2315264121. PMID: 37873283 Free PMC article. Updated. Preprint.
References
-
- Bean B. P., The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007). - PubMed
-
- Sanguinetti M. C., Tristani-Firouzi M., hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469 (2006). - PubMed
-
- Nattel S., Carlsson L., Innovative approaches to anti-arrhythmic drug therapy. Nat. Rev. Drug Discov. 5, 1034–1049 (2006). - PubMed
-
- Nichols C. G., KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

