Modeling mechanical activation of macrophages during pulmonary fibrogenesis for targeted anti-fibrosis therapy
- PMID: 38552026
- PMCID: PMC10980276
- DOI: 10.1126/sciadv.adj9559
Modeling mechanical activation of macrophages during pulmonary fibrogenesis for targeted anti-fibrosis therapy
Abstract
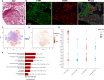
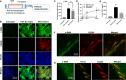
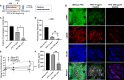
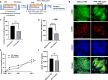
Pulmonary fibrosis is an often fatal lung disease. Immune cells such as macrophages were shown to accumulate in the fibrotic lung, but their contribution to the fibrosis development is unclear. To recapitulate the involvement of macrophages in the development of pulmonary fibrosis, we developed a fibrotic microtissue model with cocultured human macrophages and fibroblasts. We show that profibrotic macrophages seeded on topographically controlled stromal tissues became mechanically activated. The resulting co-alignment of macrophages, collagen fibers, and fibroblasts promoted widespread fibrogenesis in micro-engineered lung tissues. Anti-fibrosis treatment using pirfenidone disrupts the polarization and mechanical activation of profibrotic macrophages, leading to fibrosis inhibition. Pirfenidone inhibits the mechanical activation of macrophages by suppressing integrin αMβ2 and Rho-associated kinase 2. These results demonstrate a potential pulmonary fibrogenesis mechanism at the tissue level contributed by macrophages. The cocultured microtissue model is a powerful tool to study the immune-stromal cell interactions and the anti-fibrosis drug mechanism.
Figures


Update of
-
Modeling Mechanical Activation of Macrophages During Pulmonary Fibrogenesis for Targeted Anti-Fibrosis Therapy.bioRxiv [Preprint]. 2023 Jul 21:2023.07.19.549794. doi: 10.1101/2023.07.19.549794. bioRxiv. 2023. Update in: Sci Adv. 2024 Mar 29;10(13):eadj9559. doi: 10.1126/sciadv.adj9559. PMID: 37503121 Free PMC article. Updated. Preprint.
References
-
- Martinez F. J., Collard H. R., Pardo A., Raghu G., Richeldi L., Selman M., Swigris J. J., Taniguchi H., Wells A. U., Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers. 3, 17074 (2017). - PubMed
-
- Snijder J., Peraza J., Padilla M., Capaccione K., Salvatore M. M., Pulmonary fibrosis: A disease of alveolar collapse and collagen deposition. Expert Rev. Respir. Med. 13, 615–619 (2019). - PubMed
-
- Fabre T., Barron A. M. S., Christensen S. M., Asano S., Bound K., Lech M. P., Wadsworth M. H. II, Chen X., Wang C., Wang J., McMahon J., Schlerman F., White A., Kravarik K. M., Fisher A. J., Borthwick L. A., Hart K. M., Henderson N. C., Wynn T. A., Dower K., Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci Immunol 8, eadd8945 (2023). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

