The S-acylation cycle of transcription factor MtNAC80 influences cold stress responses in Medicago truncatula
- PMID: 38552172
- PMCID: PMC11218828
- DOI: 10.1093/plcell/koae103
The S-acylation cycle of transcription factor MtNAC80 influences cold stress responses in Medicago truncatula
Abstract
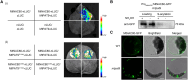
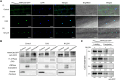
S-acylation is a reversible post-translational modification catalyzed by protein S-acyltransferases (PATs), and acyl protein thioesterases (APTs) mediate de-S-acylation. Although many proteins are S-acylated, how the S-acylation cycle modulates specific biological functions in plants is poorly understood. In this study, we report that the S-acylation cycle of transcription factor MtNAC80 is involved in the Medicago truncatula cold stress response. Under normal conditions, MtNAC80 localized to membranes through MtPAT9-induced S-acylation. In contrast, under cold stress conditions, MtNAC80 translocated to the nucleus through de-S-acylation mediated by thioesterases such as MtAPT1. MtNAC80 functions in the nucleus by directly binding the promoter of the glutathione S-transferase gene MtGSTU1 and promoting its expression, which enables plants to survive under cold stress by removing excess malondialdehyde and H2O2. Our findings reveal an important function of the S-acylation cycle in plants and provide insight into stress response and tolerance mechanisms.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

