CONTACT-01: A Randomized Phase III Trial of Atezolizumab + Cabozantinib Versus Docetaxel for Metastatic Non-Small Cell Lung Cancer After a Checkpoint Inhibitor and Chemotherapy
- PMID: 38552197
- PMCID: PMC11227305
- DOI: 10.1200/JCO.23.02166
CONTACT-01: A Randomized Phase III Trial of Atezolizumab + Cabozantinib Versus Docetaxel for Metastatic Non-Small Cell Lung Cancer After a Checkpoint Inhibitor and Chemotherapy
Abstract
Purpose: Although checkpoint inhibitors have improved first-line treatment for non-small cell lung cancer (NSCLC), a therapeutic need remains for patients whose disease does not respond or who experience disease progression after anti-PD-L1/PD-1 immunotherapy. CONTACT-01 (ClinicalTrials.gov identifier: NCT04471428) evaluated atezolizumab plus cabozantinib versus docetaxel in patients with metastatic NSCLC who developed disease progression after concurrent or sequential treatment with anti-PD-L1/PD-1 and platinum-containing chemotherapy.
Methods: This multicenter, open-label, phase III trial randomly assigned patients 1:1 to atezolizumab 1,200 mg intravenously once every 3 weeks (q3w) plus cabozantinib 40 mg orally once daily or docetaxel 75 mg/m2 intravenously once every 3 weeks. The primary end point was overall survival (OS).
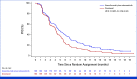
Results: One hundred eighty-six patients were assigned atezolizumab plus cabozantinib, and 180 docetaxel. Minimum OS follow-up was 10.9 months. Median OS was 10.7 months (95% CI, 8.8 to 12.3) with atezolizumab plus cabozantinib and 10.5 months (95% CI, 8.6 to 13.0) with docetaxel (stratified hazard ratio [HR], 0.88 [95% CI, 0.68 to 1.16]; P = .3668). Median progression-free survival was 4.6 months (95% CI, 4.1 to 5.6) and 4.0 months (95% CI, 3.1 to 4.4), respectively (stratified HR, 0.74 [95% CI, 0.59 to 0.92]). Serious adverse events (AEs) occurred in 71 (38.4%) patients receiving atezolizumab plus cabozantinib and 58 (34.7%) receiving docetaxel. Grade 3/4 treatment-related AEs occurred in 73 (39.5%) patients receiving atezolizumab plus cabozantinib and 58 (34.7%) receiving docetaxel. Grade 5 AEs occurred in 14 (7.6%) and 10 (6.0%) patients in the atezolizumab plus cabozantinib and docetaxel arms, respectively (treatment-related in four [2.2%] and one [0.6%], respectively).
Conclusion: Atezolizumab plus cabozantinib after disease progression following anti-PD-L1/PD-1 immunotherapy and platinum-containing chemotherapy for metastatic NSCLC did not improve OS compared with docetaxel. Safety was consistent with known profiles of these agents.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Herbst RS, Morgensztern D, Boshoff C: The biology and management of non-small cell lung cancer. Nature 553:446-454, 2018 - PubMed
-
- Hendriks LE, Kerr KM, Menis J, et al. : Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 34:358-376, 2023 - PubMed
-
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

