Long-term efficacy and safety of subcutaneous tocilizumab in clinical trials of polyarticular or systemic juvenile idiopathic arthritis
- PMID: 38552315
- PMCID: PMC11371380
- DOI: 10.1093/rheumatology/keae180
Long-term efficacy and safety of subcutaneous tocilizumab in clinical trials of polyarticular or systemic juvenile idiopathic arthritis
Abstract
Objective: To investigate the safety and efficacy of subcutaneous tocilizumab (SC-TCZ) treatment in a long-term extension (LTE) of clinical trials in polyarticular or systemic juvenile idiopathic arthritis (pJIA or sJIA).
Methods: Patients with pJIA or sJIA from two open-label, 52-week phase 1b core trials of SC-TCZ who had adequate response per investigator assessment entered the LTE and continued SC-TCZ treatment according to body weight-based dosing regimens until commercial availability or up to 5 years. Pharmacokinetics, pharmacodynamics, and efficacy were assessed for up to 3 years, and safety for up to 5 years in the LTE.
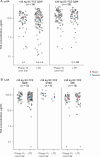
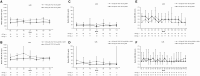
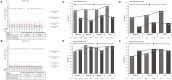
Results: Forty-four patients with pJIA and 38 patients with sJIA entered the LTE. Tocilizumab trough concentrations were maintained within the range expected to provide clinical benefit (mean values: pJIA, ∼10 μg/ml; sJIA, ∼75 μg/ml over 3 years). Pharmacodynamic parameters (interleukin-6, soluble interleukin-6 receptor, erythrocyte sedimentation rate, C-reactive protein) were maintained throughout the LTE at levels achieved in the core trials. Inactive disease per American College of Rheumatology provisional criteria was reported for 90% (17/19) and 53% (8/15) of patients with pJIA and 91% (10/11) and 92% (12/13) of patients with sJIA in the <30 and ≥30 kg body weight groups, respectively. Serious adverse events in the LTE were reported in six patients with pJIA (13.6%; five serious infections) and five patients with sJIA (13.2%; one serious infection).
Conclusion: Patients with pJIA or sJIA experienced long-term disease control with SC-TCZ treatment. Long-term safety was consistent with the known tocilizumab safety profile.
Clinical trial registration: clinicaltrials.gov, NCT02165345.
Keywords: autoinflammatory conditions; biologic therapies; clinical trial; interleukin-6; juvenile idiopathic arthritis; long-term extension; paediatric/juvenile rheumatology; pharmacology; pharmacovigilance; tocilizumab.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Martini A, Lovell DJ, Albani S et al. Juvenile idiopathic arthritis. Nat Rev Dis Primers 2022;8:5. - PubMed
-
- Ringold S, Angeles-Han ST, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken) 2019;71:717–34. - PMC - PubMed
-
- Onel KB, Horton DB, Lovell DJ et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2022;74:553–69. - PMC - PubMed
-
- De Benedetti F, Robbioni P, Massa M et al. Serum interleukin-6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin Exp Rheumatol 1992;10:493–8. - PubMed
-
- Ruperto N, Martini A. Current and future perspectives in the management of juvenile idiopathic arthritis. Lancet Child Adolesc Health 2018;2:360–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

