Embracing cancer complexity: Hallmarks of systemic disease
- PMID: 38552609
- PMCID: PMC12077170
- DOI: 10.1016/j.cell.2024.02.009
Embracing cancer complexity: Hallmarks of systemic disease
Abstract
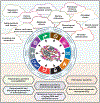
The last 50 years have witnessed extraordinary developments in understanding mechanisms of carcinogenesis, synthesized as the hallmarks of cancer. Despite this logical framework, our understanding of the molecular basis of systemic manifestations and the underlying causes of cancer-related death remains incomplete. Looking forward, elucidating how tumors interact with distant organs and how multifaceted environmental and physiological parameters impinge on tumors and their hosts will be crucial for advances in preventing and more effectively treating human cancers. In this perspective, we discuss complexities of cancer as a systemic disease, including tumor initiation and promotion, tumor micro- and immune macro-environments, aging, metabolism and obesity, cancer cachexia, circadian rhythms, nervous system interactions, tumor-related thrombosis, and the microbiome. Model systems incorporating human genetic variation will be essential to decipher the mechanistic basis of these phenomena and unravel gene-environment interactions, providing a modern synthesis of molecular oncology that is primed to prevent cancers and improve patient quality of life and cancer outcomes.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests C.A. reports employment at Saga Diagnostics and reports options to own stock in the company. C.A. and C.S. are listed as inventors on a European patent application relating to assay technology to detect tumor recurrence (PCT/GB2017/053289). This patent has been licensed to commercial entities and, under their terms of employment, C.A. and C.S. are due a revenue share of any revenue generated from such license(s). C.A. and C.S. declare a patent application (PCT/US2017/028013) for methods to detect lung cancer. C.A. and C.S. are named inventors on a patent application to determine methods and systems for tumor monitoring (PCT/EP2022/077987). C.A. and C.S. are named inventors on a provisional patent protection related to a ctDNA detection algorithm. J.A.J. has received honoraria for speaking at research symposia organized by Bristol Meyers Squibb and Glenmark Pharmaceuticals and previously served on the SAB of Pionyr Immunotherapeutics. D.H. is a founder and member of the BoD and SAB of Opna Bio, and a member of the SABs of Pfizer, Cellestia Biotech, 4D Molecular Therapeutics, and AtG Therapeutics. F.A. reports travel/accommodation/expenses from AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, and Roche, and his institution has received research funding from AstraZeneca, Daiichi Sankyo, Lilly, Novartis, Pfizer, and Roche. C.M. Consultant/Advisory fees from Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion. Principal/sub-Investigator of Clinical Trials for AbbVie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, InnatePharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, and Xencor. R.B. is founder of Oncosence. M.A.F. is founder and shareholder of Celesta Therapeutics. A.H. is a paid consultant for Calida Therapeutics. M.J.-H. has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster, Bristol Myers Squibb, and is listed as a co-inventor on a European patent application relating to methods to detect lung cancer PCT/US2017/028013), this patent has been licensed to commercial entities and, under terms of employment, M.J.-H. is due a share of any revenue generated from such license(s). J.W.L. advises Restoration Foodworks, Nanocare Technologies, and Cornerstone Pharmaceuticals. S.L. received research funding to institution from Novartis, Bristol Myers Squibb, MSD, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, AstraZeneca/Daiichi Sankyo, and Seattle Genetics. S.L. has acted as consultant (not compensated) to Seattle Genetics, Novartis, Bristol Myers Squibb, MSD, AstraZeneca/Daiichi Sankyo, Eli Lilly, Pfizer, Gilead Therapeutics, Nektar Therapeutics, PUMA Biotechnologies and Roche-Genentech. S.L. has acted as consultant (paid to institution) to Novartis, GlaxoSmithKline, Roche-Genentech, AstraZeneca/Daiichi Sankyo, Pfizer, Gilead Therapeutics, Seattle Genetics, MSD, Tallac Therapeutics, Eli Lilly, and Bristol Myers Squibb. I.M. is a consultant of LIfT BioSciences. K.M. has received honoraria from LEO Pharma, Pfizer and Bayer PLC. She has received research funding from LEO Pharma and Bayer PLC. S.Q. is a founder, CSO, and holds stock options in Achilles Therapeutics. C.S. acknowledges grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc—collaboration in minimal residual disease sequencing technologies), Ono Pharmaceutical, and Personalis. He is Chief Investigator for the AZ MeRmaiD 1 and 2 clinical trials and is the Steering Committee Chair. He is also Co-Chief Investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s Scientific Advisory Board. He receives consultant fees from Achilles Therapeutics (also SAB member), Bicycle Therapeutics (also a SAB member), Genentech, Medicxi, China Innovation Centre of Roche (CICoR) formerly Roche Innovation Centre—Shanghai, Metabomed (until July 2022), Relay Therapeutics, and the Sarah Cannon Research Institute. C.S. has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Illumina, MSD, Novartis, Pfizer, and Roche-Ventana. C.S. has previously held stock options in Apogen Biotechnologies and GRAIL, and currently has stock options in Epic Bioscience, Bicycle Therapeutics, and has stock options and is co-founder of Achilles Therapeutics. Patents: C.S. declares a patent application (PCT/US2017/028013) for methods to lung cancer); targeting neoantigens (PCT/EP2016/059401); identifying patent response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004); predicting survival rates of patients with cancer (PCT/GB2020/050221); identifying patients who respond to cancer treatment (PCT/GB2018/051912); methods for lung cancer detection (US20190106751A1). C.S. is an inventor on a European patent application (PCT/GB2017/053289) relating to assay technology to detect tumor recurrence. This patent has been licensed to a commercial entity and under their terms of employment C.S. is due a revenue share of any revenue generated from such license(s). K.H.V. is on the board of directors and shareholder of Bristol Myers Squibb and on the science advisory board (with stock options) of PMV Pharma, RAZE Therapeutics, Volastra Pharmaceuticals and Kovina Therapeutics. She is on the SAB of Ludwig Cancer and a co-founder and consultant of Faeth Therapeutics. She has been in receipt of research funding from Astex Pharmaceuticals and AstraZeneca and contributed to CRUK Cancer Research Technology filing of patent application WO/2017/144877. J.W. is an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center which covers methods to enhance immune checkpoint blockade responses by modulating the microbiome, reports compensation for speaker’s bureau and honoraria from Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, Physician Education Resource, MedImmune, Exelixis and Bristol Myers Squibb, and has served as a consultant/advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline, Bristol Myers Squibb, Micronoma, OSE therapeutics, Merck, and Everimmune. Dr. Wargo receives stock options from Micronoma and OSE therapeutics. A.W. is on the board of Regain Therapeutics.
Figures





References
Publication types
MeSH terms
Grants and funding
- R01 AG067584/AG/NIA NIH HHS/United States
- CC2051/WT_/Wellcome Trust/United Kingdom
- CC2073/WT_/Wellcome Trust/United Kingdom
- OT2 CA278665/CA/NCI NIH HHS/United States
- R01 CA243547/CA/NCI NIH HHS/United States
- OT2 CA278609/CA/NCI NIH HHS/United States
- R35 CA210263/CA/NCI NIH HHS/United States
- CGCATF-2021/100026/CRUK_/Cancer Research UK/United Kingdom
- R01 CA163591/CA/NCI NIH HHS/United States
- R35 CA210018/CA/NCI NIH HHS/United States
- R01 AI165661/AI/NIAID NIH HHS/United States
- R01 CA271500/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

