Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy
- PMID: 38552670
- PMCID: PMC12279009
- DOI: 10.1053/j.gastro.2024.03.011
Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With Blood-Based Biomarkers (Liquid Biopsy) vs Fecal Tests or Colonoscopy
Abstract
Background & aims: Colorectal cancer (CRC) screening is highly effective but underused. Blood-based biomarkers (liquid biopsy) could improve screening participation.
Methods: Using our established Markov model, screening every 3 years with a blood-based test that meets minimum Centers for Medicare & Medicaid Services' thresholds (CMSmin) (CRC sensitivity 74%, specificity 90%) was compared with established alternatives. Test attributes were varied in sensitivity analyses.
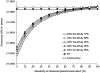
Results: CMSmin reduced CRC incidence by 40% and CRC mortality by 52% vs no screening. These reductions were less profound than the 68%-79% and 73%-81%, respectively, achieved with multi-target stool DNA (Cologuard; Exact Sciences) every 3 years, annual fecal immunochemical testing (FIT), or colonoscopy every 10 years. Assuming the same cost as multi-target stool DNA, CMSmin cost $28,500/quality-adjusted life-year gained vs no screening, but FIT, colonoscopy, and multi-target stool DNA were less costly and more effective. CMSmin would match FIT's clinical outcomes if it achieved 1.4- to 1.8-fold FIT's participation rate. Advanced precancerous lesion (APL) sensitivity was a key determinant of a test's effectiveness. A paradigm-changing blood-based test (sensitivity >90% for CRC and 80% for APL; 90% specificity; cost ≤$120-$140) would be cost-effective vs FIT at comparable participation.
Conclusions: CMSmin could contribute to CRC control by achieving screening in those who will not use established methods. Substituting blood-based testing for established effective CRC screening methods will require higher CRC and APL sensitivities that deliver programmatic benefits matching those of FIT. High APL sensitivity, which can result in CRC prevention, should be a top priority for screening test developers. APL detection should not be penalized by a definition of test specificity that focuses on CRC only.
Keywords: Blood-Based Biomarker; Blood-Based Testing; Colorectal Cancer; Comparative Effectiveness; Cost-Effectiveness; Decision Analysis; Health Economics; Liquid Biopsy; Screening.
Copyright © 2024 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
These authors disclose the following: Uri Ladabaum: advisory board of UniversalDx, Lean Medical, Vivante, and Kohler Ventures and consultant for Medtronic, Clinical Genomics, Guardant Health, Freenome, and ChekCap; Robert E. Schoen: research support: Immunovia, Exact Sciences, Freenome; David Lieberman: advisory board of UniversalDx, Geneoscopy, and Colowrap. The remaining authors disclose no conflicts.
Figures
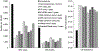

Similar articles
-
Projected Impact and Cost-Effectiveness of Novel Molecular Blood-Based or Stool-Based Screening Tests for Colorectal Cancer.Ann Intern Med. 2024 Dec;177(12):1610-1620. doi: 10.7326/ANNALS-24-00910. Epub 2024 Oct 29. Ann Intern Med. 2024. PMID: 39467291 Free PMC article.
-
Faecal immunochemical tests to triage patients with lower abdominal symptoms for suspected colorectal cancer referrals in primary care: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2017 May;21(33):1-234. doi: 10.3310/hta21330. Health Technol Assess. 2017. PMID: 28643629 Free PMC article.
-
Characteristics of a cost-effective blood test for colorectal cancer screening.J Natl Cancer Inst. 2024 Oct 1;116(10):1612-1620. doi: 10.1093/jnci/djae124. J Natl Cancer Inst. 2024. PMID: 38845072 Free PMC article.
-
Cost-Effectiveness of Noninvasive Colorectal Cancer Screening in Community Clinics.JAMA Netw Open. 2025 Jan 2;8(1):e2454938. doi: 10.1001/jamanetworkopen.2024.54938. JAMA Netw Open. 2025. PMID: 39820690 Free PMC article.
-
Chemoprevention of colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2010 Jun;14(32):1-206. doi: 10.3310/hta14320. Health Technol Assess. 2010. PMID: 20594533
Cited by
-
Identification of serum N-glycans signatures in three major gastrointestinal cancers by high-throughput N-glycome profiling.Clin Proteomics. 2024 Nov 28;21(1):64. doi: 10.1186/s12014-024-09516-2. Clin Proteomics. 2024. PMID: 39609732 Free PMC article.
-
Advances in colorectal cancer screening and detection: a narrative review on biomarkers, imaging and preventive strategies.J Egypt Natl Canc Inst. 2025 Apr 11;37(1):20. doi: 10.1186/s43046-025-00277-z. J Egypt Natl Canc Inst. 2025. PMID: 40214837 Review.
-
State of omics-based microbial diagnostics of CRC.Gut Microbes. 2025 Dec;17(1):2526132. doi: 10.1080/19490976.2025.2526132. Epub 2025 Jul 2. Gut Microbes. 2025. PMID: 40601369 Free PMC article. Review.
-
Are Non-invasive Multi-cancer Early Cancer Detection Tests the Future?Dig Dis Sci. 2025 May;70(5):1694-1702. doi: 10.1007/s10620-024-08839-2. Epub 2025 Jan 30. Dig Dis Sci. 2025. PMID: 39885052 Review.
-
Stress-Testing US Colorectal Cancer Screening Guidelines: Decennial Colonoscopy from Age 45 is Robust to Natural History Uncertainty and Colonoscopy Sensitivity Assumptions.Med Decis Making. 2025 Jul;45(5):557-568. doi: 10.1177/0272989X251334373. Epub 2025 Apr 29. Med Decis Making. 2025. PMID: 40302197 Free PMC article.
References
-
- Lin JS, Perdue LA, Henrikson NB, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325:1978–1998. - PubMed
-
- Ladabaum U, Dominitz JA, Kahi C, et al. Strategies for colorectal cancer screening. Gastroenterology 2020;158:418–432. - PubMed
-
- Centers for Medicare & Medicaid Services. Decision memo for screening for colorectal cancer - blood-based biomarker tests (CAG-00454N). Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.... Accessed November 25, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

